Source: Streetwise Reports (11/11/22)
With late-stage drug candidates and major near-term catalysts, undervalued Aldeyra Therapeutics warrants a Buy to Outperform rating and consideration by potential investors, according to various analysts.
For a biopharma with one new drug candidate on the verge of potential approval in the U.S. and a second close behind, Aldeyra Therapeutics Inc.(ALDX:NASDAQ) is currently undervalued and represents an attractive, derisked investment opportunity, experts said.
The Massachusetts-based firm develops treatments for immune-mediated diseases, which regulate entire immunological systems rather than alter a single protein. The therapeutic candidates are designed to optimize numerous pathways while limiting toxicity, and the biopharma is currently advancing three such products.
A Trio of Potential New Therapies
1) Reproxalap: This RASP, or reactive aldehyde species, inhibiting 0.25% ophthalmic solution for dry eye disease is Aldeyra’s lead drug candidate, for which the company is on schedule to file a new drug application (NDA) with the U.S. Food and Drug Administration (FDA) by year-end.
Approval “could lead to a meaningful lift for the shares,” purported Oppenheimer analyst Justin Kim.
Free Reports:
Get our Weekly Commitment of Traders Reports - See where the biggest traders (Hedge Funds and Commercial Hedgers) are positioned in the futures markets on a weekly basis.
Sign Up for Our Stock Market Newsletter – Get updated on News, Charts & Rankings of Public Companies when you join our Stocks Newsletter
In clinical trials, reproxalap was shown to be efficacious and safe. It “demonstrated robust and consistent dry eye disease benefit,” wrote H.C. Wainwright & Co. analyst Matthew Caufield in a July 13 research note. “We view RASP inhibition as presenting a viable novel pathway in addressing current dry eye disease therapeutic limitations.”
Caulfield noted that approved dry eye disease treatments on the market could take months to have an appreciable effect, have inconsistent responses among patients, and can be uncomfortable, often causing patients to stop using them. RASP is different as it is said to provide immediate relief, unlike previous therapies.
“From an FDA perspective, reproxalap is very safe, passes the Schirmer test with high significance, and has a novel mechanism of action in a field with underserved patients. We think that will be enough for approval,” said BTIG’s Thomas Shrader.
Laidlaw & Co. analyst Dr. Yale Jen stated, “Although ALDX could launch reproxalap by themselves, we believe this is a highly desirable product for large pharma companies, especially those that could leverage their existing or start an ophthalmology sales force.”
BTIG’s Thomas Shrader is one of several analysts who remain bullish on reproxalap’s chances of approval for dry eye disease. In a June 8, 2022 research report, he wrote, “From an FDA perspective, reproxalap is very safe, passes the Schirmer test with high significance, and has a novel mechanism of action in a field with underserved patients. We think that will be enough for approval.”
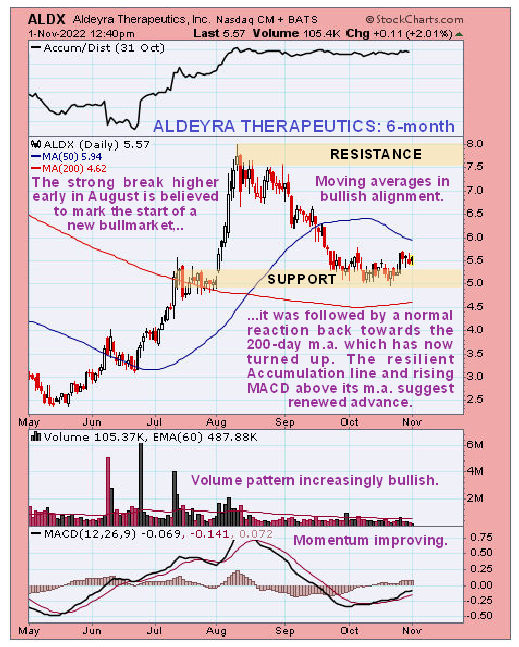
Newsletter writer Clive Maund said, in a November 1st posting, “Action since this candle looks like a tiny bull Flag suggesting renewed advance soon. Buyers here should place a stop below US$5.00.”
Reproxalap is also being evaluated for allergic conjunctivitis and is now in Phase 3.
2) ADX-2191: This intravitreal methotrexate injection is a Phase 2 developmental treatment for retinitis pigmentosa and primary vitreoretinal lymphoma. For the latter, Aldeyra has a pre-NDA meeting scheduled with the FDA this quarter.
Aldeyra intends for ADX-2191 to also prevent proliferative vitreoretinopathy (in Phase 3). Topline Phase 3 GUARD trial results suggest ADX-2191 treatment could be safer and more effective in this indication than compounded methotrexate, wrote Dr. Yale Jen, a Laidlaw & Co. analyst, in an Oct. 6, 2022 research note.
Jen also noted the current clinical package for ADX-2191 in proliferative vitreoretinopathy is “strong” and likely to support an NDA. To delineate the regulatory pathway forward for this, Aldeyra is scheduling a Type C meeting with the FDA for H1/23.
3) ADX-629. This orally administered RASP modulator is in Phase 2 clinical testing for the treatment of four immune-mediated diseases: ethanol toxicity, chronic cough, Sjögren-Larsson Syndrome, and minimal change disease.
Implications of Near-Term Catalysts
Because dry eye disease is a large, currently underserved market, FDA approval of reproxalap as a treatment for it would be a significant development for Aldeyra, BTIG analyst Shrader wrote.
Approval “could lead to a meaningful lift for the shares,” purported analyst Justin Kim in a Sept. 15 research report. His firm Oppenheimer rates Aldeyra Outperform.
Were reproxalap approved, Aldeyra could reach commercialization in 2023.
As for Aldeyra’s shares, they are currently “underexposed and undervalued,” according to Laidlaw‘s Dr. Yale Jen.
With respect to ADX-2191, positive GUARD trial results, and the pre-NDA meeting on primary vitreoretinal lymphoma, Kim noted, “could catalyze a nontrivial revenue opportunity relative to current share levels.”
As for Aldeyra’s shares, they are currently “underexposed and undervalued,” according to analyst Jen. Today Aldeyra’s share price is US$5.32, and it has been trading in the US$5 range since Sept. 20, 2022.
In comparison, Jen’s firm, Laidlaw & Co., has a US$30 per share target price on the biopharma; this represents a significant jump and return on investment from its share price today.
While Aldeyra’s cash position declined in the latest quarter, Jen noted in a November 11 research note that “ALDX ended 3Q22 with ~US$185M cash, enough to support its operations throughout 2023.” In the report, Laidlaw & Co. reiterated its Buy rating and said, “ALDX shares remain underexposed and under-valued.”
Institutions Dominate Ownership
Institutions held 68.43% of Aldeyra’s shares, the Top 3 being Perceptive Advisors LLC (16.98%), The Vanguard Group Inc. (4.18%), and Citadel Advisors LLC (3.97%). The No. 1 mutual fund holder was the Vanguard Total Stock Market Index Fund at 2.74%.
Aldeyra insiders, including Chief Executive Officer Dr. Todd Brady, Chief Development Officer Dr. Stephen Machatha, Chairman of the Board Dr. Richard Douglas, and several directors, together owned 2.36% of the company’s shares.
The Aldeyra investment opportunity is one that numerous biotech research analysts view favorably. As of them, Kim wrote, “Aldeyra’s late-stage ophthalmology pipeline in allergic conjunctivitis and dry eye diseases offers a favorable risk-reward to current share levels, coupled with long-term pipeline optionality from ADX-2191 in proliferative vitreoretinopathy and systemic RASP applications.”
Coverage and Share Structure
Aldeyra is followed by numerous analysts, including Wainwright & Co. analyst Matthew Caufield, BTIG’s Thomas Shrader, Dr. Yale Jen of Laidlaw & Co., and Justin Kim of Oppenheimer. Newsletter writer Clive Maund also follows the stock. Click “See More Live Data” in the data box above to read their reports.
Aldeyra Therapeutics’ market cap is US$320.78M. The company has 58.32 million shares outstanding, and it trades in a 52-week range of US$2.36 and US$9.06.
Disclosures:
1) Doresa Banning compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an independent contractor. She or members of her household own securities of the following companies mentioned in the article: None. She or members of her household are paid by the following companies mentioned in this article: None.
2) The following companies mentioned in this article are billboard sponsors of Streetwise Reports: None. Click here for important disclosures about sponsor fees. As of the date of this article, an affiliate of Streetwise Reports has a consulting relationship with None. Please click here for more information.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports’ terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the decision to publish an article until three business days after the publication of the article. The foregoing prohibition does not apply to articles that in substance only restate previously published company releases. As of the date of this article, officers and/or employees of Streetwise Reports LLC (including members of their household) own securities of Aldeyra Therapeutics Inc., a company mentioned in this article.
6) This article does not constitute medical advice. Officers, employees and contributors to Streetwise Reports are not licensed medical professionals. Readers should always contact their healthcare professionals for medical advice.

- The British index has updated the historical maximum. Oil lost 5% over the week May 3, 2024
- US Fed tilts towards a rate cut despite the postponement. HKMA left the rate unchanged at 5.75% May 2, 2024
- Brent crude oil hits seven-week low May 2, 2024
- Target Thursdays: USDJPY, Copper & EURCAD May 2, 2024
- WTI oil declines on rising inventories and negotiations between Israel and Hamas. Rising unemployment in New Zealand may force RBNZ to start cutting rates earlier May 1, 2024
- Bitcoin stumbles below $60k ahead of Fed May 1, 2024
- Expert Says Now Looks Like a Good Time To Buy This Renewable Energy Stock Apr 30, 2024
- Optimism over corporate earnings is fueling stock indices. The Hong Kong index reached a 5-month high Apr 30, 2024
- FXTM’s Copper: Hits fresh two-year high! Apr 30, 2024
- European indices grow on the ECB’s “dovish” position. Quarterly reports of mega-companies support the broad market Apr 29, 2024