By Wesley Chang, Drexel University
Rechargeable batteries are great for storing energy and powering electronics from smartphones to electric vehicles. In cold environments, however, they can be more difficult to charge and may even catch on fire.
I’m a mechanical engineering professor who’s been interested in batteries since college. I now lead a battery research group at Drexel University.
In just this past decade, I have watched the price of lithium-ion batteries drop as the production market has grown much larger. Future projections predict the market could reach thousands of GWh per year by 2030, a significant increase.
But, lithium-ion batteries aren’t perfect – this rise comes with risks, such as their tendency to slow down during cold weather and even catch on fire.
Behind the Li-ion battery
The electrochemical energy storage within batteries works by storing electricity in the form of ions. Ions are atoms that have a nonzero charge because they have either too many or not enough electrons.
Free Reports:
 Sign Up for Our Stock Market Newsletter – Get updated on News, Charts & Rankings of Public Companies when you join our Stocks Newsletter
Sign Up for Our Stock Market Newsletter – Get updated on News, Charts & Rankings of Public Companies when you join our Stocks Newsletter
 Get our Weekly Commitment of Traders Reports - See where the biggest traders (Hedge Funds and Commercial Hedgers) are positioned in the futures markets on a weekly basis.
Get our Weekly Commitment of Traders Reports - See where the biggest traders (Hedge Funds and Commercial Hedgers) are positioned in the futures markets on a weekly basis.
When you plug in your electric car or phone, the electricity provided by the outlet drives these ions from the battery’s positive electrode into its negative electrode. The electrodes are solid materials in a battery that can store ions, and all batteries have both a positive and a negative electrode.
Electrons pass through the battery as electricity. With each electron that passes to one electrode, a lithium ion also passes into the same electrode. This ensures the balance of charges in the battery. As you drive your car, the stored ions in the negative electrode move back to the positive electrode, and the resulting flow of electricity powers the motor.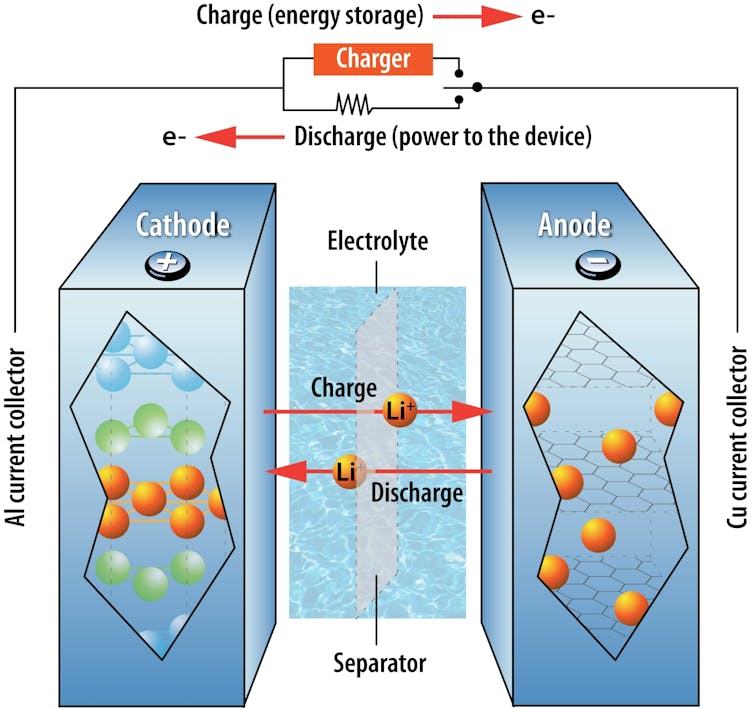
Argonne National Laboratory, CC BY-NC-SA
While AA or AAA batteries can power small electronics, they can be used only once and cannot be charged. Rechargeable Li-ion batteries can operate for thousands of cycles of full charge and discharge. For each cycle, they can also store a much higher amount of charge than an AA or AAA battery.
Since lithium is the lightest metal, it has a high specific capacity, meaning it can store a huge amount of charge per weight. This is why lithium-ion batteries are useful not just for portable electronics but for powering modes of transportation with limited weight or volume, such as electric cars.
Battery fires
However, lithium-ion batteries have risks that AA or AAA batteries don’t. For one, they’re more likely to catch on fire. For example, the number of electric bike battery fires reported in New York City has increased from 30 to nearly 300 in the past five years.
Lots of different issues can cause a battery fire. Poorly manufactured cells could contain defects, such as trace impurities or particles left behind from the manufacturing process, that increase the risk of an internal failure.
Climate can also affect battery operation. Electric vehicle sales have increased across the U.S., particularly in cold regions such as the Northeast and Midwest, where the frigid temperatures can hinder battery performance.
Batteries contain fluids called electrolytes, and cold temperatures cause fluids to flow more slowly. So, the electrolytes in batteries slow and thicken in the cold, causing the lithium ions inside to move slower. This slowdown can prevent the lithium ions from properly inserting into the electrodes. Instead, they may deposit on the electrode surface and form lithium metal.
If too much lithium deposits on the electrode’s surface during charging, it may cause an internal short circuit. This process can start a battery fire.
Making safer batteries
My research group, along with many others, is studying how to make batteries that operate more efficiently in the cold.
For example, researchers are exploring swapping out the usual battery electrolyte and replacing it with an alternative electrolyte that doesn’t thicken at cold temperatures. Another potential option is heating up the battery pack before charging so that the charging process occurs at a warmer temperature.
My group is also investigating new types of batteries beyond lithium ion. These could be battery types that are more stable at wider temperature ranges, types that don’t even use liquid electrolytes at all, or batteries that use sodium instead of lithium. Sodium-ion batteries could work well and cost less, as sodium is a very abundant resource.
Solid-state batteries use solid electrolytes that aren’t flammable, which reduces the risk of fire. But these batteries don’t work quite as well as Li-ion batteries, so it’ll take more research to tell whether these are a good option.
Lithium-ion batteries power technologies that people across the country use every day, and research in these areas aims to find solutions that will make this technology even safer for the consumer.![]()
About the Author:
Wesley Chang, Assistant Professor of Mechanical Engineering and Mechanics, Drexel University
This article is republished from The Conversation under a Creative Commons license. Read the original article.

- COT Bonds Charts: Speculator Bets led by SOFR 3-Months & 10-Year Bonds Dec 21, 2024
- COT Metals Charts: Speculator Bets led lower by Gold, Copper & Palladium Dec 21, 2024
- COT Soft Commodities Charts: Speculator Bets led by Live Cattle, Lean Hogs & Coffee Dec 21, 2024
- COT Stock Market Charts: Speculator Bets led by S&P500 & Russell-2000 Dec 21, 2024
- Riksbank and Banxico cut interest rates by 0.25%. BoE, Norges Bank, and PBoC left rates unchanged Dec 20, 2024
- Brent Oil Under Pressure Again: USD and China in Focus Dec 20, 2024
- Market round-up: BoE & BoJ hold, Fed delivers ‘hawkish’ cut Dec 19, 2024
- NZD/USD at a New Low: The Problem is the US Dollar and Local GDP Dec 19, 2024
- The Dow Jones has fallen for 9 consecutive trading sessions. Inflationary pressures are easing in Canada. Dec 18, 2024
- Gold Holds Steady as Investors Await Federal Reserve’s Rate Decision Dec 18, 2024
