By The Life Science Report
Source: The Life Sciences Report 12/21/2016
View Original Article: https://www.streetwisereports.com/pub/na/viveves-technology-for-womens-sexual-dysfunction-receives-regulatory-approval-in-49-countries
The last several months have been active ones for Viveve: The results of the VIVEVE I clinical trial were accepted for publication in the Journal of Sexual Medicine, third-quarter financial results beat expectations, and the Viveve system has received regulatory approvals in Brazil, U.A.E. and Lebanon and FDA 510(k) clearance in the U.S.
On Dec. 1, Viveve Medical Inc. (VIVE:NASDAQ) announced that the results of the VIVEVE I clinical trial were accepted in the Journal of Sexual Medicine, the peer-reviewed journal of the International Society of Sexual Medicine. According to the company, The VIveve Treatment of the Vaginal Introitus to EValuate Efficacy study is the first randomized, single-blinded and sham-controlled trial designed to demonstrate the efficacy and safety of the Viveve Treatment for the treatment of vaginal laxity, which is the result of the over-stretching of the vaginal tissue during childbirth.
Patricia Scheller, CEO of Viveve, noted, “With the publication of the VIVEVE I results, we believe that a new paradigm has been established for the use of energy-based treatments for women’s sexual health conditions. Large, randomized, sham-controlled studies have not historically been conducted to demonstrate the safety and efficacy of energy-based procedures in gynecological applications, demonstrating, I believe, a profound lack of regard for the treatment of these significant medical conditions. Viveve will be the first, and, to date, the only company to clinically demonstrate the efficacy and safety of its treatment.” The primary author of the article is Michael Krychman, M.D., executive director of the Southern California Center for Sexual Health and Survivorship Medicine.
Free Reports:
 Get Our Free Metatrader 4 Indicators - Put Our Free MetaTrader 4 Custom Indicators on your charts when you join our Weekly Newsletter
Get Our Free Metatrader 4 Indicators - Put Our Free MetaTrader 4 Custom Indicators on your charts when you join our Weekly Newsletter
 Get our Weekly Commitment of Traders Reports - See where the biggest traders (Hedge Funds and Commercial Hedgers) are positioned in the futures markets on a weekly basis.
Get our Weekly Commitment of Traders Reports - See where the biggest traders (Hedge Funds and Commercial Hedgers) are positioned in the futures markets on a weekly basis.
Viveve also released record third-quarter financial results. Analyst Jeffrey Cohen of Ladenburg Thalmann noted, “Revenues increased 217% on an annual basis and 19% sequentially as the company’s commercial activities continue to gain traction in a variety of worldwide markets with a current base of 162 systems.”
Analyst Anthony Vendetti of Maxim Group stated on Nov. 11 that Q3/16 results were ahead of expectations: “We would continue to be buyers of VIVE following its fifth consecutive quarter of double-digit revenue growth driven by the continued regulatory clearances of the Viveve System around the world. . .VIVE reported 3Q16 total revenues of $1.85M, above both our estimate of $1.65M and consensus of $1.55M.”
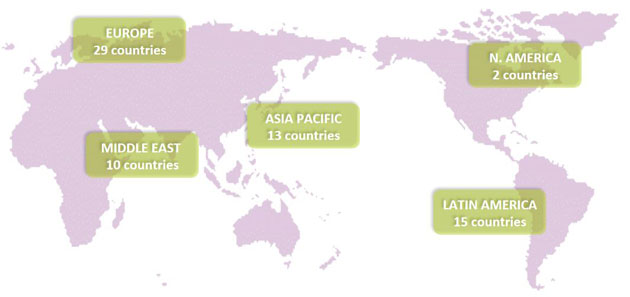
Vendetti highlighted that “3Q16 marked the company’s second quarter of commercial sales in China, and the APAC region continues to exhibit the most strength, followed by the Middle East, Europe, and Latin America, comprising roughly 65%, 25%, 6%, and 4% of sales, respectively. The company also received an FDA 510(k) clearance for electrocoagulation and hemostasis in the U.S., and it has begun commercialization.”
Brian Marckx, an analyst with Zacks Small-Cap Research, noted, “the pace of unit placements continues to accelerate as do consumables sales with Q3 marking new records in both the number of consoles and treatment tips sold as well as in total revenue. And there has been a lot of activity on the operational front as well including gaining initial regulatory clearance in the U.S. and Brazil and the September IDE submission seeking approval for the pivotal U.S.-based randomized VIVEVE II study (seeking clearance for a potential game-changing sexual function indication).”
Marckx also observed, “While the most recent quarter was impressive, additional and potentially very significant catalysts recently came online which could have the effect of steepening the already heady revenue curve. This includes regulatory clearance/approval in the U.S. (for general surgical procedures) and Brazil, both of which came in October. These two countries, which according to ISAPS rank #1 (U.S.) and #2 (Brazil) in the world for the combined number of vaginal rejuvenation and labiaplasty procedures performed each year (and combined account for ~23% of all vaginal rejuvenation and labiaplasty procedures around the world), offer fertile ground for Viveve and their newly branded Geneveve system.”
Jeffrey Cohen discussed Viveve’s U.S. strategy for its Geneveve system: “The Company has plans for dual pathways into the U.S. marketplace of which the first has been approved. In October the FDA granted a 510(k) clearance for general surgical procedures for electrocoagulation and hemostasis.” Then, “the second approach that the Company plans to pursue would entail a clinical proven pathway (trial) for approval which would likely be for the treatment of vaginal tissue to improve sexual function. We believe that the company is currently in discussion with the agency and that a formal IDE would be submitted in the coming months such that a study (VIVEVE II) would commence late in early 2017. We would anticipate that the study would take approximately 12-18 months in duration and that the company would be able to commercialize under this label in 2H-2018. The study is likely to enroll approximately 250 with 20 to 25 sites. We believe that this claim would eventually prove to further open up the potential marketplace from aesthetics to also include gynecological physicians.”
Anthony Vendetti stated, “we view the company’s recent regulatory clearances as significant milestones (i.e., S. Korea, Brazil, and the U.S.), as approval in these countries represents large market opportunities. Going forward, we believe management’s focus on attaining additional regulatory clearances and hiring a direct sales force, coupled with investments into market awareness, should position it for growth.”
Read what other experts are saying about:
Want to read more Life Sciences Report articles like this? Sign up for our free e-newsletter, and you’ll learn when new articles have been published. To see recent articles and interviews with industry analysts and commentators, visit our Streetwise Interviews page.
Disclosures:
1) Patrice Fusillo compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an employee. She owns, or members of her immediate household or family own, shares of the following companies mentioned in this article: None. She is, or members of her immediate household or family are, paid by the following companies mentioned in this article: None.
2) Viveve Medical Inc. is a billboard sponsor of Streetwise Reports. Streetwise Reports does not accept stock in exchange for its services. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports’ terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their families are prohibited from making purchases and/or sales of those securities in the open market or otherwise during the up-to-four-week interval from the time of the interview or article until after it publishes.
Additional Disclosures for this Content
Ladenburg Thalmann, Viveve Medical Inc., Nov. 14, 2016
ANALYST CERTIFICATION: I, Jeffrey S. Cohen, attest that the views expressed in this research report accurately reflect my personal views about the subject security and issuer. Furthermore, no part of my compensation was, is, or will be directly or indirectly related to the specific recommendation or views expressed in this research report, provided, however, that:
The research analyst primarily responsible for the preparation of this research report has or will receive compensation based upon various factors, including the volume of trading at the firm in the subject security, as well as the firm’s total revenues, a portion of which is generated by investment banking activities.
Ladenburg Thalmann & Co. Inc. makes a market in Viveve Medical, Inc.
Ladenburg Thalmann & Co. Inc. has managed or co-managed a public offering for Viveve Medical, Inc. within the past 12 months.
Ladenburg Thalmann & Co. Inc received compensation for investment banking services from Viveve Medical, Inc. within the past 12 months.
Ladenburg Thalmann & Co. Inc had an investment banking relationship with Viveve Medical, Inc. within the last 12 months.
Maxim Securities, Viveve Medical Inc., Nov. 11, 2016
I, Anthony Vendetti, attest that the views expressed in this research report accurately reflect my personal views about the subject security and issuer. Furthermore, no part of my compensation was, is, or will be directly or indirectly related to the specific recommendation or views expressed in this research report.
The research analyst(s) primarily responsible for the preparation of this research report have received compensation based upon various factors, including the firm’s total revenues, a portion of which is generated by investment banking activities.
Maxim Group makes a market in Viveve Medical, Inc.
Maxim Group managed/co-managed/acted as placement agent for an offering of the securities for Viveve Medical, Inc. in the past 12 months.
Maxim Group expects to receive or intends to seek compensation for investment banking services from Viveve Medical, Inc. in the next 3 months.
Zacks Small-Cap Research, Viveve Medical, Nov. 16, 2016
ANALYST DISCLOSURES: I, Brian Marckx, CFA, hereby certify that the view expressed in this research report accurately reflect my personal views about the subject securities and issuers. I also certify that no part of my compensation was, is, or will be, directly or indirectly, related to the recommendations or views expressed in this research report. I believe the information used for the creation of this report has been obtained from sources I considered to be reliable, but I can neither guarantee nor represent the completeness or accuracy of the information herewith. Such information and the opinions expressed are subject to change without notice.
INVESTMENT BANKING AND FEES FOR SERVICES
Zacks SCR does not provide investment banking services nor has it received compensation for investment banking services from the issuers of the securities covered in this report or article.
Zacks SCR has received compensation from the issuer directly or from an investor relations consulting firm engaged by the issuer for providing non-investment banking services to this issuer and expects to receive additional compensation for such non-investment banking services provided to this issuer.
The non-investment banking services provided to the issuer includes the preparation of this report, investor relations services, investment software, financial database analysis, organization of non-deal road shows, and attendance fees for conferences sponsored or co-sponsored by Zacks SCR. The fees for these services vary on a per-client basis and are subject to the number and types of services contracted. Fees typically range between ten thousand and fifty thousand dollars per annum. Details of fees paid by this issuer are available upon request.