By The Life Science Report
Source: https://www.streetwisereports.com/pub/na/17327
3D Signatures continues to advance its cutting-edge technology for prostate cancer liquid biopsies and Hodgkin’s lymphoma tests.
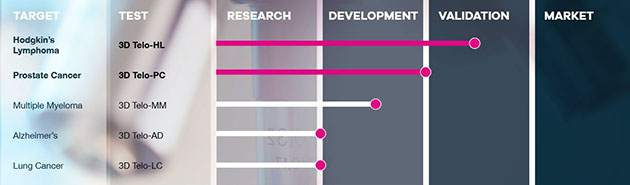
Within the past two weeks, 3D Signatures Inc. (DXD:TSX.V; TDSGF:OTCQB; 3D0:FSE) announced that it is forging ahead with the validation program for its Hodgkin’s lymphoma test, and also announced the positive results of its prospective blood-based prostate cancer pilot study.
The Hodgkin’s lymphoma test (Telo-HL) uses the company’s proprietary TeloView software program to stratify Hodgkin’s lymphoma patients “at the point of diagnosis into non-relapsing and relapsing patients so that relapsing patients may be considered for alternative treatments to standard chemotherapy at the beginning of their treatment process.” The company says that currently there is no “biomarker available that can predict patient response to standard chemotherapy in HL patients, and thereby help guide treatment decisions on an individual basis.”
Jason Flowerday, CEO of 3D Signatures, stated that “we are truly excited to initiate this program, which we believe will validate the TeloView platform as a disruptive technology based on a universal structural biomarker. Telo-HL has the unique ability to help physicians personalize effective treatments. Once commercially available, we believe this technology will give physicians foresight that is simply unavailable through conventional diagnostic testing.”
Such an ability to stratify patients would not only allow a patient to avoid a debilitating chemotherapy that will not be effective, it would also save on costs. “The ability to identify the appropriate treatment for an individual patient at the time of diagnosis should result in expedited use of alternative treatment options and cost savings to the payor,” the company stated.
The Telo-HL test is on track to be commercially marketable within 12 months.
3D Signatures also released the results of a prospective blood-based prostate cancer pilot study. For the 50 patients participating, blood was drawn and circulating tumor cells (CTCs) isolated. The company noted that in all 50 cases, “surgery results correlated with the observed three-dimensional nuclear telomeric profiles from CTCs and indicated that the TeloView platform correctly identified each patient with stable vs. progressive disease.”
The accuracy of this test offers the possibility that men with slow-growing prostate tumors could safely forego biopsies because the blood test would be able to diagnose with a high degree of certainty whether the cancer is invasive or noninvasive. The company sees TeloView as a “promising candidate for development as an accurate, blood-based risk-assessment and monitoring platform for prostate cancer.”
In addition to the pilot project, 3D Signatures’ TeloView is going to be part of PRECISE, a Canada-wide prostate cancer clinical trial that will “identify and monitor prostate cancer patients suitable for active surveillance.”
In Alphastox on March 2, Etienne Moshevich lauded 3D Signatures’ announcements: “Prostate cancer is highly overtreated and in some cases undertreated. In both cases this is due to a lack of accurate diagnostic tests. The fact that DXD’s test has the potential for this kind of accuracy from a blood test vs a biopsy is even more impressive and disruptive.” The newsletter calls the Hodgkin’s lymphoma test a “highly personalized test to improve patient outcomes and save insurance companies a lot of money. These types of tests are the future of medicine!” He added, “3D has a disruptive platform technology and there’s no doubt in my mind that they have already caught the attention of major pharmaceutical companies.”
“I see the prostate cancer and Hodgkin’s lymphoma programs as a major step forward in terms of validating the investment opportunity and driving the value creation process. DXD.V is at a strong support level with lots of good news expected over the next 6-12 months,” concluded Moshevich.
Read what other experts are saying about:
Want to read more Life Sciences Report articles like this? Sign up for our free e-newsletter, and you’ll learn when new articles have been published. To see recent articles and interviews with industry analysts and commentators, visit our Streetwise Interviews page.
Disclosure:
1) Patrice Fusillo compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an employee. She owns, or members of her immediate household or family own, shares of the following companies mentioned in this article: None. She is, or members of her immediate household or family are, paid by the following companies mentioned in this article: None.
2) 3D Signatures Inc. is a billboard sponsor of Streetwise Reports. Streetwise Reports does not accept stock in exchange for its services. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports’ terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their families are prohibited from making purchases and/or sales of those securities in the open market or otherwise during the up-to-four-week interval from the time of the interview or article until after it publishes.