- Up for debate at Fed: A sharper easy-money message (WSJ)
- Rupiah set for worst week since 2011 as central bank allows drop (Bloomberg)
- China central bank chief says to improve financing for small firms (Reuters)
- Philippines doesn’t need additional stimulus, Tetagnco says (Bloomberg)
- Japan central bank finds the pessimists come from within (Reuters)
- Central bank chief sees silver lining for Thai economy (Pattaya Mail)
- Dramatic wholesale interest rate response to OCR (Radio New Zealand)
- Chile’s central bank chief foresees lower interest rate (Xinhua)
- Jewelry exports from India to jump on new central bank rules (Bloomberg)
- Yellen or Summers, it will be Obama’s Fed (The Ticker, Bloomberg)
- Should Canada central bank chief look for more tools for the tool kit? (WSJ)
- Russia central bank: Econ risks may cap Russia econ growth, spur capital flight (Dow Jones)
- www.CentralBankNews.info
Friday Charts: Is it Time to Take a Gamble on Apple?
We’re tackling all the following questions (and a little more) in this week’s edition of Friday Charts…
- What’s the single biggest risk to Apple’s (AAPL) plan to take over the world?
- Is there such a thing as a truly all-weather investment?
- Do consumers buy more domestics or imports? (I’m talking about cars – not beer – people. Come on!)
- And will the average American ever get their finances in order?
Please enjoy the answers!
Apple: Time to Bet “on the Come”?
1%. That’s all the sales growth Apple could muster up this quarter.
Talk about pathetic. For years, the company had delivered double-digit sales and profit growth like clockwork.

Don’t fret, though. Even with shares down about 40% from their all-time high in 2012, the new(ish) CEO wants us to hang tight on the promise of better growth ahead.
In his prepared statement, Cook said, “We are laser-focused and working hard on some amazing new products that we will introduce in the fall and across 2014.”
In other words, he wants investors to bet “on the come.” I wouldn’t.
As I’ve pointed out before, the law of large numbers isn’t working in Apple’s favor. Neither are the business fundamentals.
Average selling prices (ASP) for the iPad and iPhone, which account for more than 50% of sales, are falling, not rising. The iPad ASP fell to $436 from $449 last quarter, and the iPhone ASP dropped to a historic low of $581 from $613 last quarter.
You could wait for Cook to prove that he can innovate and deliver double-digit growth via new products.
Or, better yet, there’s a way for you to collect insane amounts of cash on Apple right now. I’m talking about pocketing $25,000 or more (enough to buy a new car) – in three seconds or less. Don’t believe me? Go here to find out how it’s done.
When in Doubt, Bank on Dividends
Financial headlines always try to scare us out of one investment or another. If you’re tired of trying to make sense of all the warnings, there is a solution.
And no, it’s not going 100% into cash. Even if cash reduces your anxiety levels, it’s a stupid investment. (Want proof? Go here.)
Instead, bank on dividend-paying stocks.

Although they might lag the broader market during bull markets over short periods of time, they consistently do a portfolio good over the longer term.
Buy Domestic
Last week, I shared a shocking divergence in the real estate market. This week, I’m sharing one in the automotive market.
In the United States, new auto sales just hit the highest level in five years. In Europe, though, new auto sales dropped to their lowest levels in almost a decade.

The takeaway? Keep betting on domestic automakers, specifically Ford (F).
That’s it for this week. Before you go, though, let us know what you think of this weekly column – or any of our recent work at Wall Street Daily – by sending an email to [email protected] or leaving a comment on our website.
Ahead of the tape,
Louis Basenese
The post Friday Charts: Is it Time to Take a Gamble on Apple? appeared first on | Wall Street Daily.
Article By WallStreetDaily.com
Original Article: Friday Charts: Is it Time to Take a Gamble on Apple?
Europe shares open green ahead of earning reports
European Shares were seen opening in green on Friday, as new corporate reports from the European firms were released. Investors were supported from the higher close on Wall Street in the previous session. Shares in Europe were dropping in the previous session because of the weak earnings.
The pan-European Euro Stoxx 50 advanced 0.41% to 2,753 at market opening, while the German DAX rose 0.55% to 8,344.81. The French CAC 40 gained 0.61% to 3,980.20, while the UK FTSE 100 was up 0.39% to 6,613.50.
A batch of new reports of the European second quarter earnings are expected to be released later today, from companies such as Banco Popular, Aol and others.
Caixa Bank reported its net profits of 408 million euros during the first quarter of this year, as the bank income from investments gained a total income of 3,629 million euros.
The Automakers Renault reported its net profit for the first six months of 2013 dropped by 0.9% to 20,441 million euros , compared to the revenue 774 million euros in the first half of 2012.
The Destatis reports showed that the German import prices for the month of June dropped 0.8% month-to-month while falling 2.2% annually.
Figures fell beyond analysts had predicted in the previous month. The French consumer confidence had a slight rise to 82 in the month July from the revised 79 reported in the previous month.
The post Europe shares open green ahead of earning reports appeared first on | HY Markets Official blog.
Article provided by HY Markets Forex Blog
Most stocks in Asia opens red as China worries
Most of the stocks in the Asian market was seen closing in red for the third day in row on Friday , while Japan’s consumer prices advanced beyond expectation for the first time since April last year . China has announced the new rules which are expected to reduce excess factory capacity.
However, the decline of the ongoing earning season, have raised concerns from investors as they worry the Japanese stocks will weigh down.
The Japanese yen advanced, adding pressure on the stock in Japan as the two usually reversely correlate.
The Japanese benchmark Nikkei 225 declined 2.97%, closing at 14,129.98.91, while the Tokyo’s broader Topix gauge dropped 2.9%, closing at 1,167.06.
The Hong Kong Hang Seng traded flat, with a minor rise of 0.10% to 21,926.41. The Chinese mainland Shanghai composite declined further by 0.26% to 2,015.72.
South Korean Kospi edged up 0.06% higher to 1,910.81 on Friday, while the Australian S&P/ASX 200 index advanced 0.13% to 5,042.00.
Japan’s Consumer Price index (CPI) for the month of June ,showed an upbeat result for the first time since April last year , with a rise in prices since mid-2011.
The core CPI rose by 0.4% in June, according to the Statistics Bureau in Tokyo. While the figures exceeded predictions of an increase of 0.3% for the month of June. However, current figures are still below the 2% target made by the Bank of Japan (BoJ).
“Japan is gradually shifting to inflation from deflation,” Japan’s Finance Minister Taro Aso said confidently following the readings that “CPI data shows the overall trend is improving little by little.”
While in China , investors continues to worry about the Chinese economy , as the Chinese Ministry of Industry and Information Technology ordered more than 1,400 companies in 19 industries to decrease their excess capacity by September this year .
On Thursday, the preliminary Purchasing Managers Index indicted that the Chinese factories are trapped with contraction due to the fall in the contraction territory in July.
The Chinese leadership announced on Wednesday, that the railway development project along with a reform is aimed to improve the business for small companies and reduce fees for exporters.
The post Most stocks in Asia opens red as China worries appeared first on | HY Markets Official blog.
Article provided by HY Markets Forex Blog
USDCAD remains in downtrend from 1.0442
USDCAD remains in downtrend from 1.0442, and the fall extends to as low as 1.0255. Resistance is now located at the upper line of the price channel on 4-hour chart. As long as the channel resistance holds, the downtrend could be expected to continue, and next target would be at 1.0200 area. On the upside, a clear break above the channel resistance will indicate that the downtrend from 1.0442 is complete, then the pair will find resistance around 1.0360.
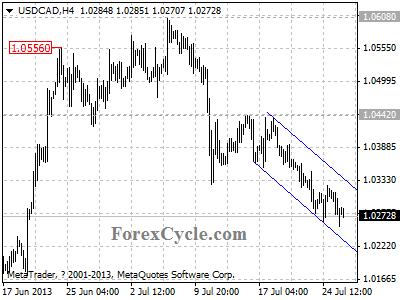
Provided by ForexCycle.com
Is This the Spark to Send Australian Property Crashing?
If you want to make a splash in the Australian market, just do one thing – talk about property.
We found that out to our cost when we tried to explain to Australians that housing isn’t a magic investment…that as someone with experience in the stock market, we know that asset prices don’t always go up…
Sometimes they go down too. Take for instance the folks in Queensland who built a house for $21.5 million, which the bank seized when the owners defaulted, and has just sold for…$5 million.
Of course, one terrible property investment doesn’t make a crash. In fact, seven years after property prices began to crash in the US and Europe, Australian house prices are almost back to the 2010 peak.
What does this mean? Did Australia miss the housing crash? Or is it simply waiting in the wings…?
According to Property Observer:
‘Melbourne was the strongest performing capital city housing market over the June quarter with house prices rising 5% to $553,447 to sit just 1.4% below their previous peak recorded in June 2010, according to figures compiled by Australian Property Monitors (APM).‘
A buoyant market goes towards backing controversial economist Phillip J Anderson’s view that Aussie housing is on the verge of a 14-year bull market.
Anderson says the Australian housing market follows the US housing market and the Dow Jones Industrial Average. But his analysis isn’t just from a few years of stock market returns.
He uses numbers going back 300 years. It’s all part of his super-cycle theory. And at the moment it’s hard to argue with it…
Biggest Housing Bubble Grows Bigger?
You only have to look at the cold hard numbers.
The US housing market bottomed out in 2011 and has started to rebound. The US stock market has (after a few bumps) recovered all the ground it lost during the 2008 crash…and added some.
And even the slowcoach Australian stock market has piled on good gains, especially during the past year.
Yet quietly, almost without notice it seems Australian house prices have mounted a comeback too. As APM says, Melbourne house prices – the market most feared to be in a price bubble – are within 1.4% of the 2010 all-time high.
So, what do we make of it?
Well, the fact remains that asset prices never go up forever. That’s one thing proved time and again despite the best efforts of bubble deniers.
But this move has us stumped. Is it really possible that Phil Anderson is right? Is the Australian market really at the beginning of a 14-year bull market run?
Not so fast, says our old pal, editor of The Denning Report, Dan Denning…
This is a Retail Story Not a Property Story
Yesterday morning Dan showed us an article in the Australian:
‘First it was the supermarket owners, then the Lowys. Now the Commonwealth Bank of Australia is seemingly distancing itself from the local property market, with a proposal to sell the management rights of its $20 billion real estate investment trusts and some wholesale assets to boot.‘
Dan has a reputation for picking a trend early. The prime example was the shale gas craze, which Dan has followed since 2005. That was a good six years ahead of mainstream Aussie analysts, who only started following the industry in 2011.
So, does this spell trouble for Australian property? Maybe. But there’s more to this story than meets the eye.
For a start, CBA’s flogging-off of property assets has nothing to do with the residential real estate market. It’s a commercial property deal.
But as Dan notes, this isn’t just about property. The bigger picture is the broader Australian economy – to be precise, the retail sector.
That’s the thing, CBA’s move is just as much about lack of confidence in retailing as it is about property. The reason for that is tenants in shopping malls have to pay the landlord a percentage of sales turnover on top of a fixed rent.
But with retail sales hitting the skids, clearly the real estate trusts aren’t getting the same cash flows they used to get. You can see why. The Australian Bureau of Statistics reported May consumer spending only climbed 0.1%. That was below analyst estimates.
The only other option is to raise rents. But if the sales aren’t there, that risks tenants moving elsewhere or just plain shutting up shop for good.
A Unique Retail REIT Play
There’s no doubt real estate investment trusts (REITs) are risky right now. Actually, that’s not true. In June 2011 we backed an Aussie REIT in Australian Small-Cap Investigator that used a different model.
We said it was one of the only (perhaps the only) retail REIT on the Aussie market that was a pure property play.
You see, unlike other retail REITs, it doesn’t charge tenants a percentage of turnover. It simply charges a fair market rent based on rents for similar properties in the area.
And that’s it.
Sure, this pure property play doesn’t get the cream when the retail sector booms. But that means it doesn’t lose a revenue stream when the retail sector isn’t booming…like now.
But that’s OK, because it still gets to collect the rent. Add to this the fact that its chief tenant is a blue-chip Aussie brand name and it’s hard to see how things can go wrong.
That said, this pure property play is unique (as far as we’re aware) in the Aussie market. That’s why we’re not bothered what happens to the Australian property market or the retail sector.
But if you own other commercial REITs all we can say is beware. Dan smells trouble in the sector, and based on CBA’s decision to downsize its REIT exposure it looks as though he’s on to something.
Cheers,
Kris+
Visit the Remembering The Future Facebook Page for more on Phil Anderson.
From the Port Phillip Publishing Library
Special Report: The Sixth Revolution
Daily Reckoning: Productive Investments
Money Morning: Why the Australian Share Market Could Reach 7,000 by 2015…
Pursuit of Happiness: Foreign Family in Taxpayer Rort…Or Royal Celebration?
Australian Small-Cap Investigator:
How to Make Big Money from Small-Cap Stocks
New Australian Home Buyers Aren’t Convinced
[Ed: This originally appeared in The Daily Reckoning on the 14/5/2013]
The key to blowing up a successful asset bubble is that you must constantly attract new money into the asset class you’re trying to inflate.
By that standard, recent Australian housing finance figures were better than expected but worse than required. The numbers were up. But new home buyers have not yet been bullied into the market by lower rates.
The value of home loans for owner-occupied housing rose 5.8% to $14.9 billion in March, according to the figures from the Australian Bureau of Statistics.
This was a firm rejection of our prediction that the numbers would suck. To be more specific, let’s put it in the form of a question: have rate cuts put a housing-led recovery back on the cards?
The numbers would have been welcome news to Glenn Stevens and the team at the Reserve Bank of Australia. They know Australia needs lower rates and more business investment to compensate for lower commodity prices and China’s shift to a consumption model. But there’s more to this housing data than meets the eye.
First, new home buyers aren’t convinced. New home buyers made up only 14.2% of demand. That’s the lowest percentage in nine years. What does it tell you?
Low interest rates are nice. And lower interest rates may be even nicer. But no matter how often a brain-damaged economist repeats it, lower interest rates don’t make a $600,000 house more affordable for someone on a $60,000 income. They just mean you’ll have to borrow more money now and repay it for longer in order to have a roof over your head.
What WAS interesting about the data is the big jump in new construction loans. They were up over 10% on the month and over 21.4% from the same time last year. This is a result of state governments creating incentives for new home buyers to actually build rather than buy. As public policy, it’s designed to increase housing stock, which should eventually actually lead to lower house prices.
That bit caught our eye because it suggests that some people are a lot more interested in building houses than, say, buying stocks. We’ve been working with our friend Phil Anderson on a project that explains and forecasts Australian property prices. The latest bit of data may confirm Phil’s view that Australia is actually on the verge of an 18-year boom in property prices.
That view certainly came as a shock to us when Phil first articulated it. But it’s based in part on the idea that land values move in cycles. Those cycles are determined by the availability of credit created by the banks and the willingness of people to borrow money. Phil has put the argument together in a presentation you can view here.
Look Out For This
In the meantime, we have to say it’s certainly not our view. In Austrian economic terms, more investment in Australian property at these prices is simply a continued misallocation of resources based on an irrational view that property always goes up.
There’s also the usual myth that Australians value housing more highly as a social goal than other countries, which has nothing to do with how ridiculously unaffordable prices still are.
But in a red pill/blue pill way, Mr Anderson’s views may make sense. That is, if you’re giving up on shares as an asset class to grow or preserve your wealth, you still have to do something with your money. Investment in land is really the only viable option for the middle class. At least it’s tangible.
And let’s consider what would happen if Australian interest rates were zero-bound. If the RBA lowers rates to around 2% in order to spur business investment, you’d expect to see a surge in non-bank lenders offering low-rate, high loan-to-value mortgages to anyone with a pulse.
You can argue whether it’s a good idea to be deliberately imitating the US-subprime boom, given how disastrous that was for everyone involved. But it doesn’t mean it won’t happen anyway.
In any event, even though we find Phil’s ultimate conclusions controversial, we were impressed with the depth of his work on property cycles. Phil brings in the work of Nickolai Kondratiev and WD Gann as well. As a publisher, this is exactly the type of well thought out market research we’re keen to publish in Australia.
Dan Denning+
Editor, The Denning Report
Visit the Remembering The Future Facebook Page for more on Phil Anderson.
From the Archives…
Why Invest ‘Hard’ When You Can Invest ‘Easy’?
19-07-2013 – Kris Sayce
Read This Before You Buy Another Stock or Bond…
18-07-2013 – Murray Dawes
Could Uranium be the Best Investment in 2013?
17-07-2013 – Dr Alex Cowie
Asteroid Mining and the Commercialisation of Space
16-07-2013 – Sam Volkering
Why the Australian Share Market is Heading Even Higher
15-07-2013 – Kris Sayce
Is Cell Therapy the ‘Future of Medicine’?: Jason Kolbert
Source: George S. Mack of The Life Sciences Report (7/25/13)
http://www.thelifesciencesreport.com/pub/na/15472
The pricey evolutionary tradeoff for walking on two legs is the curse of lower back pain. Jason Kolbert of the Maxim Group understands the power of stem cells as disease-modifying therapies for degenerative disc disease of the spine, a leading-edge, multibillion-dollar indication. Kolbert explores the opportunities that cell therapies offer investors in this interview with The Life Sciences Report, and puts a personal spin on their regenerative promise.
The Life Sciences Report: Jason, I wanted to ask you some questions about degenerative disc disease (DDD) and the use of cell therapy to manage the problem. Some people have said that the spine is a hostile environment. Why is it hostile?
Jason Kolbert: It is a unique environment versus other areas of the body because of the relative dearth of vasculature. In critical limb ischemia (CLI), congestive heart failure (CHF), stroke and other diseases there is a significant amount of blood flow and a lot of metabolic movement to the affected areas. The spine is hostile because it does not have the same level of blood flow and metabolic activity as other tissues.
TLSR: Could that minimal amount of metabolic processing in the disc space mean that cells have a longer time to work if placed directly into lesions?
JK: It’s the opposite of that. When we inject cells into the intervertebral space, we want those living cells to be enriched with a good blood supply because they must react to the local environment. It is important for the cells to survive long enough in the diseased space to exert their therapeutic effects. In the hostile spinal environment, one could argue that cells either have to be more robust or that they must exert their effects in a shorter period of time because of their shorter life expectancy. That’s been one of the problems in using cell therapy in the spine.
TLSR: Briefly, tell me about DDD.
JK: The intervertebral space contains a cartilaginous disc that, under normal and healthy conditions, cushions two neighboring vertebrae and enables normal, pain-free articulation of the spine in the upright human. However, with advancing age there is a progressive loss of the proteoglycan material that confers these stress-mitigating properties on the disc.
Let me address two types of treatment, and I don’t want them to be confused. In dealing with herniated, bulging and generally degenerated discs, we want to stop the pain by stopping the inflammation and hopefully, in a best-case situation, healing the diseased tissue. In a spinal fusion, a direct connection between two vertebrae is made, stopping their ability to move and rub against each other, and thus stopping the potential for nerve encroachment and pain. The two are often linked—that is, disc disease often precedes a fusion (especially if the fusion is not accident- or cancer-related).
TLSR: Endpoints are how we measure efficacy. What is an acceptable endpoint for degenerative disc disease? Clearly, we want the patient to be pain-free, but is that enough?
JK: We, as analysts, are asked that question a lot. Pain can be a subjective endpoint, so whenever we don’t have to use it—and can use a qualitative endpoint instead—we prefer to avoid it. That said, from the U.S. Food and Drug Administration’s (FDA’s) point of view, reduction of pain is an acceptable endpoint and does represent the standard by which it has and will likely continue to approve drugs for DDD.
Secondary endpoints are important as well, and evidence of those may be observed via MRI or radiographic imaging. This is where we get into a lot of nuance. Mesoblast Ltd. (MSB:ASE; MBLTY:OTCPK) ran preclinical studies with its human mesenchymal precursor cells (MPCs) in ovine (sheep) models. Sheep are quadrupeds, resting and walking on four legs. Gravity does not exert the same vertical or upright force on a quadruped as it does on a bipedal mammal like a human. In Mesoblast’s ovine studies there was beautiful radiographic evidence of restoration of vertebral height.
Now researchers want to know if restoration of vertical height should or could be a primary endpoint for Mesoblast’s DDD trial in humans. We don’t think it should because of the inherent differences between two-legged and four-legged mammals. It simply doesn’t make sense to use radiographic imaging of vertical height a primary endpoint, but it could be a secondary endpoint. We also think it may take more time to show radiographic changes versus changes in pain patterns. In terms of a clinical trial and product development strategy, it makes more sense to use pain as the primary endpoint. That is what we expect Mesoblast, and other companies that follow them, to do.
TLSR: Lack of–or diminished–pain is certainly going to be enough from a patient’s perspective. But obviously researchers want to be able to tie in radiographic and/or MRI changes in the same way imaging evidence is used in cancer drug development. Correct?
JK: I would stop you right there. I would not mix cancer and DDD trials. They’re very different. For cancer, the gold standard is to delay mortality—to increase overall survival. In degenerative disc disease, the primary endpoint that the FDA will use is reduction in pain. However, if pain reduction in a specified period of time is supported by qualitative data such as improvement shown in radiographic imaging evidence, that would be the Holy Grail of spinal disease.
TLSR: I’ve dwelled on imaging evidence because pain is very subjective. In the management of chronic or intractable pain, clinicians might give patients a written test every three months to measure their pain-reaction threshold.
JK: That is a great point. As I previously mentioned, as an analyst, I am nervous when pain is a primary endpoint because I want to know how pain was measured and how the trials were run. Were they double-blinded? Were they placebo-controlled? Trial design has to be scrutinized.
However, the reality is that in a disease like DDD, pain reduction is the standard primary endpoint. Can you take someone who’s had degenerative disc disease over a period of 15 to 40 years and reverse the damage? That would be asking cells to do a lot. A trial should not fail if a patient becomes pain-free but doesn’t demonstrate the imaging changes we’d love to see. The reality is, if you can alleviate the suffering from degenerative disc disease and avoid the progression to spinal fusion, then the value you bring to the patient–and the reduction in costs to the system–are huge.
TLSR: One more question about trial design in DDD: In cancer trials we are bound, in most instances, to begin with standard of care—older, approved treatments—before progressing to an experimental drug. In the meantime, patients have become more resistant to any therapy. Can you relate that to Mesoblast’s human studies?
JK: Yes. Mesoblast did something very interesting in its phase 2 human clinical trial for DDD. It recruited steroid-refractory patients—people who had already failed steroids for their pain. Therefore, the fact that cells showed a good result in these patients is very significant. We think the long-term use of steroids does nothing toward healing disc disease. Cell therapy opens up an entire new treatment paradigm in back pain.
TLSR: Does cell therapy have to be superior to current therapies, steroids in particular? Isn’t it enough that cells won’t cause steroid necrosis in the spine?
JK: Mesoblast went around the current therapy issue entirely by enrolling steroid-refractory patients in its trial. If it can demonstrate that cell therapy works in patients who have failed steroids, the company is dealing in a niche market, essentially an unmet medical need. That’s huge.
Going forward, once Mesoblast has FDA approval, how would it extend the label for frontline usage? That’s the question. Would it have to run a control arm against steroids and show noninferiority? That’s more likely than being required to show superiority, particularly if it occurs in a sequence where it has already shown utility in steroid-refractory patients. That is the brilliance of the Mesoblast strategy.
Provided a pivotal trial replicates the phase 2 data I would expect to see NeoFuse (immunoselected, culture-expanded, nucleated, allogeneic MPCs), Mesoblast’s spinal fusion cell therapy, approved and on the market as early as 2016. NeoFuse represents a highly cost-effective alternative to open spinal surgery for patients, and would target patients whose discs have degenerated too far for any hope of repair, where fusion is the only viable option to eliminate pain. In other words, cell therapy creates a new alternative for treating DDD patients. Surgery is always an option and is reserved for when less-invasive options have failed.
TLSR: Let’s go to how the cells work. Is engraftment of cells necessary for therapeutic effect?
JK: This is misunderstood by most people in the space, who believe engraftment relates to autologous cells (derived from the same patient to which they will be administered) versus allogeneic cells (from a same-species donor). The fact is that engraftment is not occurring with Mesoblast’s allogeneic cells, but neither is engraftment occurring with most autologous cell therapies.
When we talk about indications in which cell therapies might be useful, such as degenerative disc disease, cardiovascular disease or critical limb ischemia, we’re talking about cells acting like microfactories, producing therapeutic proteins or cytokines for a period of time before going away. It’s not a question of autologous or allogeneic. This is a key point. We don’t believe that engraftment is part of the cell therapy paradigm for the most part. It’s a question of the cell’s ability to react with potency to the local environment, and not a question of one cell type versus another.
TLSR: You are, in essence, saying that there is a paracrine or druglike effect with these microfactories, and then the cells leave. That implies that retreatment will be necessary in the future. In my mind, that could be a limiting factor in the use of allogeneic materials, such as Mesoblast’s cells. I find it interesting that these DDD studies involve a single administration of cells, not multiple doses over time. Is there still an overhang of potential danger in the readministration of these allogeneic cells? The immune system could mount a huge immune response or defense against their reintroduction into a patient.
JK: The answer is no. We have seen no tangible evidence that the readministration of allogeneic cells puts you at risk for any type of anaphylaxis.
We spent a lot of time with Mesoblast and Athersys Inc. (ATHX:NASDAQ) looking at the historical examples and the number of treated patients. We found zero evidence of immune response. We’re aware of the recent events with Pluristem Therapeutics Inc. (PSTI:NASDAQ), which uses full-term maternal placenta-derived adherent stromal cells. The FDA placed a hold on the company’s 74-patient phase 2 study for intermittent claudication/peripheral artery disease due to a serious allergic response that required hospitalization of one patient in the trial. We believe that is a Pluristem-specific issue.
Let’s delve into the immune reaction topic because it is also key. Manufacturing is critical. What are you actually doing to the cells? After they are harvested from the body, do you purify them? Do you enrich them? Do you expand them? Even autologous cell companies are manipulating cells in some way. They may qualify as minimally manipulated, but they’re still manipulated, whether an allogeneic cell or an autologous cell. Therein lies the opportunity to create the basis for an immune reaction. If a cell has been exposed to a particular serum or a contaminant in the manufacturing process, or if it has been roughly treated or exposed to turbulent flow, that contaminant or process may impact cell vitality and create an immune reaction.
You were at the Alliance for Regenerative Medicine Investor Day conference back in April. I was a panelist, and Maxim Group was one of the co-hosts. When NeoStem Inc. (NBS:NYSE.MKT) was finishing up its presentation, it suggested that autologous cells were safe. By inference the company suggested that allogeneic cells were not. We believe that is an example of misdirection, because reality tells us that it’s a question of manufacturing.
The questions are: Does a company have a robust manufacturing process with proper controls? Does the company know, in fact, that the process is safe? In its animal work, Mesoblast went so far as to manufacture both autologous and allogeneic cells to see if there was any difference in immune response. If there were a difference, it could be clearly traced back to the cell itself. And the company found no difference—no immunotoxicity. Mesoblast was validating the robustness of its manufacturing process. So long as a company has that, it will be OK. No one should use Pluristem’s hiccup and the FDA’s clinical hold as evidence that allogeneic is not safe.
TLSR: In other words, an immune reaction might be attributed to an artifact introduced into the patient, not the cells themselves.
JK: Correct. It may be an artifact introduced by the manufacturing process that Pluristem has to track down and correct.
We believe that other autologous companies face the same risks in their manufacturing processes. If we look at Baxter International Inc. (BAX:NYSE), with its autologous CD34+ mesenchymal stem cells from peripheral blood, or at NeoStem with its CD34+ bone marrow-derived autologous cells, these companies are enriching the cells using columns in the manufacturing process. The cells are separated, sorted and flow through the column. They interact with the column and the resulting fraction is enriched for a specific cell marker. The cell product, as such, has been manipulated. Each step is an opportunity for something to go wrong, such as the introduction of a contaminant. Starting with an autologous cell does not mean that the final product is safe.
I will grant that there are probably fewer manufacturing steps in an autologous setting, but the individualized handling, lack of automation and quality control are all limited by comparison to the advantages of a well-defined allogeneic process. If there is any manipulation whatsoever, the risk of anaphylaxis exists.
TLSR: I’d like to talk about administration of cells. Is each company developing its own methods and cannula systems to administer the cells, or will there be an all-purpose device to administer cells for intervertebral disc disease?
JK: I believe companies will develop their own devices. In fact, BioRestorative Therapies Inc. (BRTX:OTCBB) has developed a very interesting cannula that seems to work extremely well and is very unique. Companies are trying not to change the traditional methodology of the pain treatment paradigm for disc disease. So cells will be introduced in the same way that steroids are introduced. However, cells are not steroids, and it makes sense that a specialized cannula is needed.
One of the things that caught my eye is that BioRestorative Therapies is doing work with cells from brown fat. It’s an interesting argument, and a different cell source than Mesoblast’s. But the company has developed a lot of anecdotal data in humans that suggests its cells are like Mesoblast cells—robust and with potential therapeutic value in bulging and herniated disc disease.
TLSR: What is your impression of BioRestorative versus Mesoblast?
JK: BioRestorative is an interesting little company with mountains of anecdotal data that suggest its cells and delivery device may actually work. Having looked at Mesoblast’s data in DDD and in spinal fusion, it looks like it works too. The question is: If BioRestorative follows Mesoblast, where will that leave it if Mesoblast is the first mover? Only the management teams are in the position to answer.
Suffice to say that 5–10 years from now, I believe there will be competing cell therapies for disc disease. There will be an optimum cell type and delivery methodology for each disease or situation. But that thinking is too sophisticated for where the industry is today. It comes down to this: Right now the outlook for cell therapy is binary—either it works or it doesn’t work. I believe the evidence is clear—cell therapy can work, in the right indication, at the right “dose,” with the right cell type and in the right trial. Mesoblast has the resources in place to address these issues and will, I believe, end up with a positive outcome. Once the market accepts the reality—once we have an approved blockbuster cell therapy—the natural next question will be how we make the therapy better. My bet is that there will be multiple competing products, each with respective pros and cons.
TLSR: The anesthesiologists, the interventional neurologists and the neurosurgeons who treat spinal disease are already injecting drugs like steroids into intervertebral spaces. Are payers going to look at these cell therapies as just another drug going into a patient’s intervertebral space and ultimately want to pay on that basis?
JK: That’s exactly the right question. The payers are going to ask why should they pay for cell therapy at, let’s assume, the $10,000 per dose level if that will give the patient the same level of pain relief that could be achieved with a steroid at $100 per dose. That is going to be an issue. It’s going to be important for companies like Mesoblast and BioRestorative Therapies to generate data suggesting not only that cell therapy works, but also that it works in patients where steroids don’t—and that cell therapy does what steroids can’t, which is to arrest disease progression. Steroids treat the symptoms and not the underlying problem, and there is good reason to believe that cell therapy can not only alleviate pain, but also help arrest disease progression. The value of that is huge.
As far as the cost of spinal fusion. . .now we’re talking tens of thousands of dollars, and fusion often doesn’t even stop the pain. Sometimes a fusion of two vertebrae creates problems in adjacent vertebrae. Most clinicians and patients see fusion as a last resort. Clearly the treatment armamentarium needs a new resource. To the extent that a company like Mesoblast or BioRestorative can show data that changes the paradigm, payers will see value.
TLSR: Jason, you have addressed the issue that companies like Mesoblast and BioRestorative Therapies are trying not to change the way clinicians perform procedures. Clearly, administration of cells is not technique-sensitive relative to what clinicians are already doing. Uptake should be pretty rapid if these therapies are proven efficacious, shouldn’t it?
JK: You’ve hit the nail on the head. If you ask the neurosurgeon or the pain specialist to learn a new modality or technique for administration of an agent, you’re talking about a steeper learning curve, and a longer time to penetrate the marketplace. If clinicians don’t have to change their procedures in any way, shape or form, you’re talking rapid uptake.
I think the latter will be the case with cell therapies in degenerative disc disease and even spinal fusion, which might also be accomplished with cells. In fact, in the case of spinal fusion, cell therapy may actually make the orthopedic surgeon’s life easier because a step is eliminated. The surgeon is not performing an autologous bone graft from the hip, which can be very painful for the patient. If you can keep the paradigm identical—or even eliminate a step—we think there is a huge win for the patient, the clinician and the product developers.
Eliminating a step is where we believe allogeneic therapy wins. First, it tends to have lower cost of goods versus autologous, and second, it is patient friendly. You don’t have to harvest cells or tissues from the patients—not from their bone marrow, not from their fat, not from their peripheral blood. An allogeneic therapy is off-the-shelf and ready to use. That’s true even when we look at CHF or STEMI (ST-segment elevation acute myocardial infarction)-based CHF treatment, where Athersys is working.
For instance, in the catheterization laboratory, when a stent is being placed, allogeneic therapy would be readily available without harvesting anything from the sick patient, and the patient would not have to return for a second procedure. You want to limit the number of interventions you make to a patient who has heart disease. As investors scrutinize companies, it’s very important to recognize which score best on the standard SWOT (strengths, weaknesses, opportunities and threats) analysis.
TLSR: Have we seen response related to dose of cells?
JK: The answer is yes, but the reasons are not obvious. When we look at small molecules, we expect to see a linear response. You give dose one at 5 mg, dose two at 10 mg, dose three at 15 mg and dose four at 20 mg, and expect to see increasing efficacy and maybe increasing adverse events until a maximum tolerated dose and efficacy plateau is reached.
Cell therapy, on the other hand, doesn’t follow a linear curve. You have to put in enough cells to hit critical mass, but you could use too many cells, with the result being lower efficacy. Let me explain. Let’s say there are three doses of cells, 5 million (5M), 10M and 20M. It may be that 5M cells are not enough to exert the therapeutic benefit because we did not reach critical mass. But it could be that 20M cells are ineffective because too many cells end up competing for resources in a confined space and/or in a hostile environment. You could overload that space, with the result being that the cells don’t survive long enough to exert a therapeutic effect. You could ultimately determine that 10M cells are optimal. By the time researchers get to phase 3, they should have a really good handle on the right dose for a given indication.
TLSR: Jason, these cell therapy companies have not moved like biotech companies over the past year. Why are they not participating in what has been a broader biotech rally?
JK: There is inefficiency in the micro-cap space. Not only is there inefficiency, but there aren’t many analysts who follow cell therapy. Most analysts don’t really understand it.
When we look at the larger-cap companies that are heavily followed by institutions on both the buyside (institutional money management) and sellside (investment bank-driven research), there is much greater efficiency in the trading of shares. I’ll give you a great example. At Maxim we recently launched coverage of Teva Pharmaceutical Industries Ltd. (TEVA:NASDAQ). When I was at the Teva research and development conference, I counted about 400 analysts in the room. Most of them were on the buyside. As for the sellside, I don’t believe we’ve ever seen an analyst who covers Teva write extensively about its partnership with Mesoblast. It’s very clear that there is a very large inefficiency in the marketplace.
The challenge for many stem cell companies is how good data will change the company. Mesoblast is moving into pivotal trials for spinal fusion and degenerative disc disease, and also into a large, 1,700-patient trial in CHF that is being paid for by Teva—to the tune of $130M. I think it’s very important that investors step back and ask themselves what is real and what’s not real.
TLSR: Teva owns 20% of Mesoblast, and that equity is now worth $300–350M to Teva. Even with Teva’s sponsorship, Mesoblast is down 18% over the past 52 weeks.
JK: Let me add a few things. Mesoblast is primarily domiciled in Australia, and its shares are very thinly traded in the U.S. Even with a $1.8B market cap and $330M in cash on its balance sheet, it is not well followed.
We’re not going to get real institutional ownership in cell therapy stocks until they have data and proof of concept. If you’re a fund manager with $10 billion ($10B) under management, you can’t afford to buy a micro-cap company or even a big-cap company that is thinly traded in the U.S. Companies like Mesoblast need to establish a more significant U.S presence that will lead to better share liquidity, which will then allow institutional ownership. Then I believe institutions will say, “OK, if I own Mesoblast, what else might work?” They will start scouring the landscape to find companies like Osiris Therapeutics Inc. (OSIR:NASDAQ), Athersys or Pluristem, which are allogeneic counterparts to Mesoblast. The fund managers can then ask, “Are these stocks cheaper alternatives?”
TLSR: By the way, how is your ankle?
JK: Thank you for asking. I’m not just an analyst; I’m also a patient of Dr. Steven Victor, founder and CEO of IntelliCell BioSciences Inc. (SVFC:OTCPK). As you remember, I rotated my ankle this past winter with extensive damage to the ligaments. I was evaluated at the emergency room and had an orthopedic specialist follow up. The original prognosis was 3–6 months of recovery with minimal physical stress, such as running. Dr. Victor treated my ankle and for me, an n=1, the results were amazing.
Truly, the treatment was as close to minimally manipulated cell therapy as one can get. A stromal vascular fraction was extracted from my belly fat. The harvest was 750M cells; 50M were injected locally into my ankle, with 700M administered systemically. Dr. Victor believes that the systemic administration of cells in conjunction with the local administration creates two modalities of repair. The local repair process accelerates and is supported by the systemic impact, which address generalized inflammation.
I will tell you, my ankle was close to 100% within 10 days of treatment. I had a fantastic season of skiing this past winter, and have been water skiing all summer long. Ankle strength is critical in water skiing.
Clearly, I personally believe cell therapy works. I believe it’s viable. I know that from my personal experience, and while I’m only one patient, the results were dramatic. I think cell therapy represents the future of medicine.
TLSR: The therapy that you had administered did not have to be approved by the FDA. There is anexemption for minimally manipulated biological tissues. No premarket approval (PMA) or even 510(k) clearance is necessary. Correct?
JK: That is exactly correct. In the case of IntelliCell BioSciences, cells are extracted from adipose tissue, but there is no collagenase (collagen enzyme) added to digest it. The company essentially appliessonification (ultrasound) and separates out the stem cells. Within an hour it reintroduces the cells to the patient. Because cells are so minimally manipulated and because the entire process is done onsite, in the room adjacent to the patient, it likely qualifies under the FDA exemption that allows physicians to treat patients at their discretion as a “practice of medicine” issue.
Plastic surgery centers around the world are discarding patients’ fat. But we would say that there is gold—your stem cells—in that fat. I know that Dr. Victor has treated more than 300 patients, and he’s had some amazing successes. These are not FDA-controlled trials. They’re not blinded. They’re not randomized. He is one of many clinicians finding ways to harvest stem cells and treat patients with them, with amazing results. But I want to be very clear: I’m not promoting the use of stem cells without clinical trials. Clinical trials are the only pathway for the industry to commercialize the value of this technology. That said, the evidence is building that our bodies possess the ability to heal with a little bit of coaxing from good science.
TLSR: I have one last question. What’s to stop an anesthesiologist or neurosurgeon or orthopedist who treats back pain from extracting a stromal vascular fraction and treating their patients? How is that going to affect Mesoblast and others who have gone the long route with the great expense of clinical trials?
JK: There is nothing to stop people from doing that. But the caveat is that once a product is approved and has a label, things change. Imagine that two patients are treated, one with the Mesoblast product and one with the do-it-yourselfer. Let’s say something goes wrong with the do-it-yourselfer, and that patient’s pain is not ameliorated. It gets worse, and a fusion is required. The patient calls a lawyer, and the lawyer sues the doctor. The lawyer asks the doctor why he used an “unapproved do-in-yourself” therapy versus the FDA-approved product. That’s not a position I would want to be in as a treating clinician.
Once there is an approved therapeutic project, the do-it-yourselfers tend to go away. Therefore, we don’t see these as a fundamental threat to companies like Mesoblast or Cytori Therapeutics Inc. (CYTX:NASDAQ) because once clinical trials are completed and the product is approved, clinicians will use it.
TLSR: As always, it’s a pleasure speaking with you.
JK: It’s a pleasure to talk with you. Thank you.
Jason Kolbert has worked extensively in the healthcare sector as product manager for a leading pharmaceutical company, a fund manager and as an equity analyst. Prior to joining Maxim Group, where he is managing director, Kolbert spent seven years at Susquehanna International Group, where he managed a healthcare fund and founded SIG’s biotechnology team. Previously, Kolbert served as the healthcare strategist for Salomon Smith Barney. He is often quoted in the media and is a sought-out expert in the biotechnology field Prior to beginning his Wall Street career, Kolbert served as a product manager for Schering-Plough in Osaka, Japan. He received a bachelor’s degree in chemistry from State University of New York, New Paltz, and a master’s degree in business administration from the University of New Haven.
Want to read more Life Sciences Report interviews like this? Sign up for our free e-newsletter, and you’ll learn when new articles have been published. To see a list of recent interviews with industry analysts and commentators, visit our Streetwise Interviews page.
DISCLOSURE:
1) George S. Mack conducted this interview for The Life Sciences Report and provides services to The Life Sciences Report as an independent contractor. He or his family own shares of the following companies mentioned in this interview: None.
2) The following companies mentioned in the interview are sponsors of The Life Sciences Report:Athersys Inc., NeoStem Inc., BioRestorative Therapies Inc. Streetwise Reports does not accept stock in exchange for its services or as sponsorship payment.
3) Jason Kolbert: I or my family own shares of the following companies mentioned in this interview: NeoStem Inc. I personally am or my family is paid by the following companies mentioned in this interview: None. I was not paid by Streetwise Reports for participating in this interview. Comments and opinions expressed are my own comments and opinions. I had the opportunity to review the interview for accuracy as of the date of the interview and am responsible for the content of the interview.
4) The following companies are investment banking clients of Maxim Group: Athersys Inc., Cytori Therapeutics Inc. Maxim Group or its affiliates have received compensation from the following companies in the past 12 months: Mesoblast Ltd., Athersys Inc., Pluristem Therapeutics Inc., Cytori Therapeutics Inc.
5) Interviews are edited for clarity. Streetwise Reports does not make editorial comments or change experts’ statements without their consent.
6) The interview does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports’ terms of use and full legal disclaimer.
7) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned and may make purchases and/or sales of those securities in the open market or otherwise.
Streetwise – The Life Sciences Report is Copyright © 2013 by Streetwise Reports LLC. All rights are reserved. Streetwise Reports LLC hereby grants an unrestricted license to use or disseminate this copyrighted material (i) only in whole (and always including this disclaimer), but (ii) never in part..
Streetwise Reports LLC does not guarantee the accuracy or thoroughness of the information reported.
Streetwise Reports LLC receives a fee from companies that are listed on the home page in the In This Issue section. Their sponsor pages may be considered advertising for the purposes of 18 U.S.C. 1734.
Participating companies provide the logos used in The Life Sciences Report. These logos are trademarks and are the property of the individual companies.
101 Second St., Suite 110
Petaluma, CA 94952
Tel.: (707) 981-8204
Fax: (707) 981-8998
Email: [email protected]
Meet High Risk and High Reward, Biotech’s Profit Partners: Bert Hazlett
Source: George S. Mack of The Life Sciences Report (7/25/13)
http://www.thelifesciencesreport.com/pub/na/15464
Small-cap biotech investing carries real risk. Startup companies tend to have only a few ideas in development, which leaves ample room for setbacks—even room for disaster. Robert “Bert” Hazlett, senior biotechnology research analyst with ROTH Capital Partners, doesn’t mind taking such risks, but tempers them with a diversification strategy designed to minimize the impact of potential hiccups and blowups. In this interview with The Life Sciences Report, Hazlett expands on his strategy and mentions four companies with experienced management teams and high hopes for huge returns.
The Life Sciences Report: Bert, you have longevity in the business as a sellside analyst, and I thought it would be worthwhile to get your thoughts on how biotech investing has changed over the last 15 years.
Bert Hazlett: It’s been quite a rollercoaster. For more than 15 years now, I’ve been considering the drug development industry broadly—both on the biotechnology side and the pharmaceutical side. From an investing perspective it seems as if it’s been either feast or famine. However, at this point, I believe some of the fundamentals for the industry have changed for the better, and I am more constructive on the investment prospects for the life sciences sector than I have been in a long time.
When you step back and view it broadly, this is the first time in well over a decade that there has been a whiff of efficiency gains in the area of drug development. These gains, I believe, are due to the progression in our understanding of the biology of dysregulated cells, combined with greater regulatory acceptance of biomarkers and other tools that together are leading to enriched, more definitive trial designs. For these reasons, individual companies and their programs have the potential to progress more rapidly and more efficiently.
These developments are encouraging, some of the first positive steps we have seen toward efficiency improvements in drug development in a very long time, and we’ve seen positive responses in terms of the biotech and pharmaceutical indices as a result. I believe these efficiency gains will be increasingly recognized and will continue to permeate the investment climate for the next several years.
TLSR: Bert, do retail investors need to understand the science behind a therapy, or do they just need to understand the unmet need and the size of the market?
BH: I believe they need a bit of both. Retail investors need to understand that an unmet need is significant, and they need to understand the particular strategy being used by a company or therapeutic to address that unmet need. There has to be at least a basic understanding of the biology—and though it would help, that understanding doesn’t necessarily need to be exhaustive.
What we have encouraged investors to do is to consider more of a portfolio approach toward investing in the biotechnology sector. In drug development, complications inevitably arise. Sometimes they can be anticipated, sometimes not. Sometimes they’re small, and sometimes they’re more meaningful. They can create material issues for companies, some of which are solvable and some of which are not. To mitigate the impact of a specific complication with any single program, I believe a portfolio of assets is the way to proceed in biotech investment.
TLSR: Bert, we have both institutional and retail readership at The Life Sciences Report. I want to ask one more question from the retail point of view. Have retail investors begun to get a handle on the complexities of biotech investing? Do they understand the inherent risks, especially as they look at animal models and expect those data and that efficacy to be translated to humans?
BH: I think it’s important for retail investors—and all investors—to understand that increasingly complex leaps of faith and judgment are necessary for successful drug development. The process involves preclinical work, where researchers look for safety and evidence of proof of principle, but it becomes increasingly complex as research gets into humans. To some degree, you have to have faith in a management team’s ability to successfully interpret, understand and apply the nuances learned from research to move a compound from preclinical models to humans, and then to successfully position the compound so it has the best opportunity to demonstrate efficacy and safety in a manner that will make it competitive in the marketplace. A retail investor needs to come to grips with the fact that biotech is a high-risk area. And I can’t stress this enough—a portfolio approach is essential to diversify risk.
TLSR: Now that you have firmly established the need for a portfolio approach to biotech investing, why don’t you pick a basket of stocks for our readers? Start with your favorite one.
BH: To make those leaps of faith and span the gaps in efficacy between animal models and humans, an investor should select companies led by either successful investors or management teams with particular expertise in an area. Along those lines, let me start with two names: OvaScience Inc (OVSC:OTCBB) and Prothena Corp. PL C (PRTA:NASDAQ).
OvaScience is a company focusing on fertility, an area that has not seen a lot of innovation over the past 15–20 years. The company’s management team has been able to develop opportunities on the back of very intriguing recent biological discoveries. OvaScience co-founder and CEO Michelle Dipp, a physician, is also a founder and partner at the Longwood Fund, which establishes and invests in healthcare companies. Before OvaScience, she was with Sirtris Pharmaceuticals, which was acquired byGlaxoSmithKline (GSK:NYSE) back in 2008 for $720 million ($720M). She was also involved in the sale of the company. You have a leader at the OvaScience helm who is very seasoned in medicine, as well as in biotechnology and pharmaceutical development.
OvaScience is relying on the recent emergence of biology that shows mature females have egg-producing cells, which the company calls EggPCs. These EggPCs are the source of OvaScience’s new technologies, which are designed to enhance procedures like in vitro fertilization (IVF).
TLSR: I know the company has two programs. Would you talk about them and the value proposition?
BH: OvaScience’s first program, AUGMENT, is based on supplementing unfertilized eggs with additional energy in the form of mitochondria from EggPCs, which can increase the success rate of IVF. The therapy is designed to improve IVF by injecting mitochondria from EggPCs into a mature oocyte, along with sperm. If successful, this would be a very important development in the world of fertility. The company could launch AUGMENT as early as H2/14, on the heels of a study that is currently underway. AUGMENT has the ability to be a material addition to the IVF process.
The company has a second technology, called OvaTure, which takes EggPCs and actually matures them into fertilizable eggs. This is groundbreaking technology that could actually change or replace IVF. That said, OvaTure is at an earlier stage and is a higher risk technology. Right now, the AUGMENT product alone makes OvaScience very attractive. We have an $18 price target. We like OvaScience a lot.
TLSR: Are any hormones used in the AUGMENT process to stimulate production of these EggPCs?
BH: The typical IVF process includes ovarian hyperstimulation, producing a larger number of eggs using a hormone injection. The small ovarian tissue biopsy taken to harvest the EggPC material with AUGMENT occurs prior to the IVF procedure, so no hormones are used beyond what is required for regular IVF.
TLSR: The regulatory pathway for the AUGMENT program is the U.S. Food and Drug Administration’s (FDA’s) 361 HCT/P pathway, which is for minimally manipulated tissues and cells. Is there any chance the FDA could step in and say it will regulate this technology and require trials under a premarket approval (PMA), or the less stringent 510(k) pathway?
BH: The 361 HCT/P deals with specific tissues and minimal alteration of those particular tissues. Our view is that AUGMENT is eligible for 361 HCT/P because it is using the mitochondria from a patient’s own cells, though there is a risk that the FDA could ask the company to do more work than its current single study. The company has communicated with FDA about its plans for AUGMENT, and at this point has not been asked to do more.
TLSR: Investors are obviously interested in share price catalysts. As we move into and through H2/13, the company is scheduled to expand its study. Could this be a catalyst to move the shares in a positive direction?
BH: The upcoming study expansion is important, and one of the reasons the company raised money recently. I believe that increased awareness that OvaScience is moving aggressively into certain international jurisdictions should be a catalyst for investors. Particularly in Europe, where there is a much higher rate of IVF procedures, there has been good receptiveness to OvaScience and AUGMENT.
TLSR: What is the business model of OvaScience? How will the company realize revenue from the AUGMENT program when it’s marketed? What is its product?
BH: The separated mitochondria isolated from the EggPCs are the company’s product. OvaScience has not disclosed a ton of information about its process because it considers that a trade secret.
TLSR: When the product is returned to the clinician, the procedure then becomes a regular IVF procedure. Is that right?
BH: When the mitochondrial product is returned to the clinician, the intra-cytoplasmic sperm injection occurs, as does the supplemental injection of the mitochondria into the egg. AUGMENT largely fits right into the regular IVF regimen.
TLSR: I’m sure most payers will not be covering this service. Fertility has traditionally been a cash business. How much will this cost the patient?
BH: We believe that pricing would be about the same as that of acquiring a donor egg, which is roughly $15,000 ($15K) per procedure. OvaScience’s view is that it should be able to price its technology along those lines because individuals would likely prefer to successfully use their own eggs.
TLSR: Are additional doses able to be preserved until the next ovulation cycle? Will multiple doses of mitochondrial material be sent back to the obstetrician/gynecologist?
BH: Both the cells and the mitochondria can be preserved. OvaScience has done cryo (freezing) tests on the mitochondria, and they appear active after freezing. There is the potential for multiple doses to be created, but we are not certain how that will be handled by the company.
TLSR: AUGMENT is the lead program for OvaScience. It’s in the clinic, in a trial of up to 40 patients who have failed 2–5 IVF cycles. The program will utilize the 361 HCT/P pathway, so basically, the company does not need FDA approval. On the other hand, the preclinical OvaTure program, which is designed to create mature fertilizable eggs from the patient’s EggPCs, will require an investigational new drug (IND) process and ultimately a new biologic license application (BLA) must be filed. Why the difference?
BH: With AUGMENT, OvaScience is taking a mature fertilizable egg and adding to its level of mitochondria. With OvaTure, it is taking the EggPCs themselves and maturing them in a bed of ovarian tissue to become fertilizable oocytes. This program is still in early stages, and in our view there are materially more regulatory hurdles to be completed. Because of that, it is higher risk. But it is being viewed by investors—and by me—as a potential replacement for IVF rather than a cooperative technology.
TLSR: You said you wanted to talk about Prothena. Go ahead.
BH: Like OvaScience, Prothena has a seasoned management team that includes experts in drug development who came from Elan Corp. (ELN:NYSE). The Prothena team played a material role in the development of anti-abeta (amyloid beta protein) antibodies for Alzheimer’s disease and of the anti-cell adhesion antibody Tysabri (natalizumab) being used in treatment of multiple sclerosis (MS). The team at Prothena has significant expertise in the areas of protein misfolding and cell adhesion, which are the focus of the company’s primary development efforts. We think the company represents a high-risk/high-reward opportunity for investors, given management’s expertise and the opportunities within its pipeline.
TLSR: I’m looking at the development pipeline, and the disease indications are all tough ones. The lead compound NEOD001, an antibody, for AL and AA amyloidosis, is in phase 1, and there are preclinical programs for PRX002 for Parkinson’s disease and PRX003 for inflammatory diseases and metastatic cancer. None of the Prothena programs are palliative—they are all proposed as disease-modifying agents. If any one of these programs can be developed to maturity, your target price of $16 is very low.
BH: We agree. If any one of these programs progresses materially, our current target price will turn out to be very low. We incorporate healthy discounts in all of these programs because, as you correctly point out, these are disease-modifying opportunities for very significant, very devastating conditions that are also very high risk.
Our view is that Prothena has good shots on goal for all of these indications. That doesn’t guarantee success by any stretch of the imagination, but the Prothena team has developed antibodies that have shown activity in other challenging settings. We believe this team understands protein misfolding extremely well, and can develop therapeutics with the potential to be quite meaningful.
Regarding the lead program, NEOD001 for amyloidosis, some investors may say it’s very early. We are interested because, in this initial study, the antibody is being examined in patients with amyloidosis, rather than in healthy volunteers, so we may get a meaningful signal of efficacy even at this early stage. The antibody is designed to clear the misfolded protein deposits in these patients, and because of that, we could see some benefits in organ performance. If the therapy shows even modest benefit organ or disease burden, we believe that could trigger the potential for its rapid development.
TLSR: Are you saying that a phase 3 program might not be necessary in this situation?
BH: Absolutely. Amyloidosis is a disastrous situation for patients. With a meaningful signal in this initial study, if this antibody behaves in humans as it has in preclinical models, you could envision a scenario where you have very rapid development of the molecule. A meaningful phase 2 study might be enough. Having that potential, with signals that can be gleaned from early-stage data—perhaps as early as the beginning of next year—puts Prothena in a different class than an ordinary biotech company with a phase 1 program.
TLSR: When will we hear about the phase 1 program, since it could be so important?
BH: We hope to have early data on NEOD001 in the beginning of next year, and the potential to move into phase 2 could occur as early as H1/14.
Prothena’s other programs are worth considering as well. PRX002 for Parkinson’s disease is an antibody that targets misfolded alpha synuclein, which appears to be a bad actor in neuronal death in Parkinson’s. That target is gaining a lot of attention in the field, and Prothena may enlist a partner to move the program along rapidly, which we believe would be a positive catalyst for its shares.
PRX003, for inflammatory diseases and metastatic cancer, targets the melanoma cell adhesion molecule (MCAM)—right up the alley for this team, which developed Tysabri for MS. MCAM is an extremely interesting target, as it is involved with tumor angiogenesis and is a recognized marker of cancer metastasis, so the company has several directions to consider with its development. Though it is very early, the more I consider this product’s potential, the more I am intrigued, and I believe the company feels the same way.
TLSR: Let’s move to another idea, please. What else did you want to talk about?
BH: Endocyte Inc. (ECYT:NASDAQ) is a very interesting company that is evaluating the use of prognostic tools to identify patients who have the potential to respond better to its oncology therapy. It is using overexpression of the folate receptor, which is expressed on tumor cells but not on most healthy cells, as a biomarker. Between 80–90% of ovarian and lung cancers express the receptor. Endocyte has developed a folic acid vehicle linked to a cytotoxic warhead that targets the folate receptor. The folic acid/cytotoxic conjugate binds to and is internalized by the folate receptor through endocytosis, and becomes very damaging to the particular tumor cells. Ovarian tumors show the greatest folate receptor expression, and that setting is the initial indication for both the diagnostic and therapeutic.
TLSR: How is the prognostic/diagnostic used?
BH: Etarfolatide is a radiolabeled isotope combined with folic acid that binds to the folate receptor, which functions as an imaging agent used to identify folate receptor-positive tumors or sites.
TLSR: The tumor is illuminated, so to speak, with the imaging agent, and that is the indication for the use of the proposed product vintafolide (EC145), which is now in phase 3 for platinum-resistant ovarian cancer? Is that right?
BH: Exactly. The diagnostic agent etarfolatide (EC20) is given, the scan is performed, and the question is how positive the response is. It is a relatively simple procedure. And the first indication for the therapeutic vintafolide is the platinum-resistant ovarian setting, as you mentioned. I believe it has a good chance for conditional approval for that setting in the EU later this year, based on its phase 2 data. Importantly, that approval would also validate other folate receptor-based programs in the company’s pipeline.
TLSR: Is the company selecting out the high expressers?
BH: That is exactly what’s happening. About 40% of patients are high expressers of folate receptor in refractory ovarian cancer, and the goal is to identify them. However, the diagnostic also screens out those who do not have folate receptor-expressing tumors, those that are not good candidates for vintafolide. So the procedure basically screens out 60% of patients. We think that this approach will appeal to entities like the European Medicines Agency (EMA), and various governments that are going to consider paying for treatment in Europe. We think payers in the U.S. will respond to this as well.
TLSR: What’s the next catalyst for Endocyte?
BH: Etarfolatide (the imaging agent) and vintafolide (the therapeutic agent) are now being examined by the European authorities. The combined application for both the diagnostic and therapeutic was filed with the EMA last October, and the agency will make a decision in the next several months as to whether or not it is going to approve this product conditionally. Data readouts for a phase 2b study in non-small cell lung cancer and a phase 3 study in ovarian cancer are also expected during the first half of 2014.
TLSR: In April 2012, Endocyte partnered vintafolide with Merck & Co. Inc. (MRK:NYSE), which now has an exclusive license to develop, manufacture and commercialize the product. However, Endocyte is responsible for conducting the phase 3 PROCEED study and the phase 2b TARGET trial for non-small cell lung cancer (NSCLC).
BH: The deal is interesting from both parties’ perspectives. Endocyte got a large chunk of money up front—$120M. The trial design and protocol were largely underway for the phase 3 ovarian study in the U.S., so Endocyte is managing that study, with Merck funding a portion of it. Beyond that, Merck is funding all of the lung cancer work and the other studies, even when Endocyte is conducting the trial. There are also potential milestones worth another $880M to Endocyte, plus the U.S. profit share, plus an international double-digit royalty.
Interestingly, Endocyte has retained full economics to the etarfolatide diagnostic, which could help with development down the road of more potent therapeutics with folic acid as the targeting agent. All in, we think the partnership is a very good deal for Endocyte.
TLSR: Can you talk about one more name? I know you are following BioLineRx Ltd. (BLRX:NASDAQ). Some results are expected late this year.
BH: I view BioLineRx as an incubator for very interesting technologies that are sourced from Israel and other international geographies. Again, in line with my management theme, the company has a team that’s canny in terms of developing novel opportunities in various therapeutic areas. BioLineRx’s business model is to take products from the early clinic or preclinical stage and develop them through proof of concept—into phase 2, let’s say—and then license them to others who will develop the products further.
BioLineRx has a collection of different assets. An interesting one is BL-8040 (formerly BKT-140), a CXCR4 antagonist that’s being considered for acute myeloid leukemia (AML). This molecule appears to have CXCR4 receptor-binding characteristics that result in direct apoptotic (cell death) activity. BL-8040 is the company’s first opportunity in oncology. It’s a challenging indication, but we’re hopeful that initial results, due around year-end, can demonstrate activity in AML.
TLSR: This product, BL-8040, is not the lead program at the company, is it?
BH: It is not. The lead compound, BL-1040, was out-licensed to Ikaria Inc. (private). BL-1040 is in late-stage development for post-acute myocardial infarction (AMI). You can think of it as an injected support matrix that provides mechanical support to myocardial tissue that has been damaged. The support matrix is established by the calcium that is present in the damaged myocardium, and the matrix then erodes over time. It’s in a CE Mark registration trial now, which should have results sometime in the middle of next year. Again, the technology is higher risk, but has a pretty high reward. The company also has very interesting opportunities in hepatitis C and in dermatology, which are expected to advance during 2014.
TLSR: Bert, this company’s business plan is to be a technology incubator. Is the idea to get other companies to front the big cash outlays for late-stage development and then reap a royalty stream?
BH: That’s exactly the point. BL-1040 is now in someone else’s hands. There is the potential for BL-8040 to also be licensed out in the not-too-distant future, assuming some beneficial responses are demonstrated in AML. Being an incubator with multiple shots on goal in multiple therapeutic areas I think should have appeal for investors. The business model creates the necessity for different competencies within the management team. The team not only has to be good at developing drugs, but it also has to be good at out-licensing them.
TLSR: Bert, thank you for the time today. I enjoyed it all very much.
BH: Thank you.
Robert (Bert) Hazlett, a biopharmaceuticals senior research analyst, joined ROTH Capital Partners in 2012. Hazlett has more than 15 years of equity research experience covering the biopharmaceuticals sector. Prior to joining ROTH, Hazlett was a managing director and senior analyst at BMO Capital Markets, covering both the large capitalization and emerging pharmaceuticals sectors. Before BMO, he covered similar sectors as a managing director at SunTrust Robinson Humphrey, as a vice president at Robertson Stephens, and also held analyst positions in healthcare research at Lehman Brothers and UBS Securities. Hazlett received his master’s degree in business administration (finance) in 1996 from Columbia University and a bachelor’s degree in economics in 1987 from Yale University.
Want to read more Life Sciences Report interviews like this? Sign up for our free e-newsletter, and you’ll learn when new articles have been published. To see a list of recent interviews with industry analysts and commentators, visit our Streetwise Interviews page.
DISCLOSURE:
1) George S. Mack conducted this interview for The Life Sciences Report and provides services to The Life Sciences Report as an independent contractor. He or his family own shares of the following companies mentioned in this interview: None.
2) The following companies mentioned in the interview are sponsors of The Life Sciences Report: Merck & Co. Inc. Streetwise Reports does not accept stock in exchange for its services or as sponsorship payment. Merck & Co. Inc. is not affiliated with Streetwise Reports.
3) Bert Hazlett: I or my family own shares of the following companies mentioned in this interview: None. I personally am or my family is paid by the following companies mentioned in this interview: None. My company has a financial relationship with the following companies mentioned in this interview: OvaScience Inc., BioLineRx Ltd. I was not paid by Streetwise Reports for participating in this interview. Comments and opinions expressed are my own comments and opinions. I had the opportunity to review the interview for accuracy as of the date of the interview and am responsible for the content of the interview.
4) Interviews are edited for clarity. Streetwise Reports does not make editorial comments or change experts’ statements without their consent.
5) The interview does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports’ terms of use and full legal disclaimer.
6) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned and may make purchases and/or sales of those securities in the open market or otherwise.
Streetwise – The Life Sciences Report is Copyright © 2013 by Streetwise Reports LLC. All rights are reserved. Streetwise Reports LLC hereby grants an unrestricted license to use or disseminate this copyrighted material (i) only in whole (and always including this disclaimer), but (ii) never in part..
Streetwise Reports LLC does not guarantee the accuracy or thoroughness of the information reported.
Streetwise Reports LLC receives a fee from companies that are listed on the home page in the In This Issue section. Their sponsor pages may be considered advertising for the purposes of 18 U.S.C. 1734.
Participating companies provide the logos used in The Life Sciences Report. These logos are trademarks and are the property of the individual companies.
101 Second St., Suite 110
Petaluma, CA 94952
Tel.: (707) 981-8204
Fax: (707) 981-8998
Email: [email protected]
Capture Upside in Undervalued, Underfollowed Energy Stocks: Peter Epstein
Source: Rita Sapunor of The Energy Report (7/25/13)
http://www.theenergyreport.com/pub/na/15475
Big gains are rarely found by jumping on the bandwagon. Independent Analyst and founder of MockingJay Inc., Peter Epstein argues that market darlings won’t reward latecomers; that’s why he spends his time finding undervalued, underfollowed junior resource companies. In this interview with The Energy Report, Epstein shares his resource stock diamonds in the rough, including a uranium name commercializing a groundbreaking technology and a graphite company beating its competitors to market.
The Energy Report: You’ve written that “a great company doesn’t necessarily make a great investment.” Are you implying juniors are better bets?
Peter Epstein: Yes—juniors are highly risky in that they can move up or down by quite a large amount, but if, for example, a junior is trading at its cash value, how risky is it really? At some point the upside outweighs the risk. Make no mistake: You still need good projects, cash in the bank and a strong management team, but if you’re buying shares in a company that’s trading below its cash value, you’re basically getting its assets for free.
TER: What’s an example of a great company that’s not necessarily a great investment?
PE: Look at Cameco Corp. (CCO:TSX; CCJ:NYSE), a true leader in the uranium space and a great company. If underlying uranium prices rebound to $75 or $85 per pound ($85/lb), as many pundits expect, analyst price targets indicate that Cameco’s stock might increase by about 25%. However, select oversold juniors in the space could return multiples of the amount invested.
TER: Your portfolio really runs the gamut of resource sectors. How do you choose which commodities to focus on?
PE: I read a lot and I speak with many management teams and industry experts. It’s not necessarily difficult to pick the commodities that have strong core fundamentals. For example, iron ore fundamentals look challenging, as Rio Tinto (RIO:NYSE; RIO:LSE; RTPPF: OTCPK), BHP (BHP:NYSE; BHPLF:OTCPK), Vale (VALE:NYSE) and Fortescue Metals (FMG:ASX), together representing two-thirds of the iron ore industry, are ramping up production levels dramatically.
On the other hand, uranium fundamentals appear quite strong. Japan is restarting a number of nuclear reactors early next year and China and India are building new reactors as fast as they can. China has little choice. Its major cities are choked with coal-related air pollution, not to mention the millions of new cars on its roads. India’s coal market is hopelessly complicated and corrupt. Coal-fired electricity generation there can’t possibly keep up with demand. India’s stated goal is to get 25% of its power from nuclear energy, from which it currently gets just 3%. All of this suggests increasing demand for uranium.
TER: What are some oversold juniors you’re watching in the uranium space?
PE: Energy Fuels Inc. (EFR:TSX; EFRFF:OTCQX) is extremely well positioned in the U.S. It will have two of the top-five uranium development projects in the U.S. once it closes on its announced acquisition ofStrathmore Minerals Corp. (STM:TSX; STHJF:OTCQX).
Energy Fuels owns the only operating conventional uranium mill in the country. This mill has a replacement cost in the hundreds of millions, yet Energy Fuels’ fully diluted market cap is just $125 million ($125M). Energy Fuels trades at a very substantial discount to peer uranium producers. The last time there was a major bull market in uranium stocks, Energy Fuels’ stock was up by 400% within about nine months. This time around, the stars are aligning for big gains once again.
TER: How do you determine the top-five U.S. projects? Is it based solely on size?
PE: It’s largely based on the scale of the projects. The U.S. is home to a number of emerging in-situ recovery (ISR) projects that should produce 2–8 million pounds (2-8 Mlb) of resource over the next four to eight years. These are companies like Ur-Energy Inc. (URE:TSX; URG:NYSE.MKT), Uranerz Energy Corp. (URZ:TSX; URZ:NYSE.MKT) and Uranium Energy Corp. (UEC:NYSE.MKT).
The difference with Energy Fuels is that its projects are conventional mining operations as opposed to ISR, which some believe is a lower-cost method. However, if you have a conventional mining project that’s three times as large as an ISR project, you’re still going to make strong returns at that scale, even if it’s at a lower margin. To be clear though, the economies of scale of Energy Fuels’ major projects could easily even out the margins as compared to proposed ISR projects.
TER: Black Range Minerals Ltd. (BLR:ASX) has an unusual uranium ore concentrating technology called ablation. How does that method measure up to an ISR or conventional mining project?
PE: The technology concentrates uranium mineralization at the mine site by 90% or more by separating waste from ore in a low-cost, green, purely mechanical process. Therefore, instead of shipping 100 tons of ore to a mill that could be hundreds of miles away, only 10 tons of concentrate need be shipped. This translates into immense savings at every step of the mining operation. Ore is cheaper to transport and process and there are 90% less tailings!
The unique thing about Black Range Minerals is that in addition to its Hansen/Taylor Ranch uranium project in Colorado, which, at 91 Mlb, makes Black Range a top-five resource holder in the U.S., the company also has a 50/50 joint venture (JV) with a private company named Ablation Technologies LLC. This JV has exclusive global rights to ablation technology, which could be a game-changer. The majors will be watching the deployment of a semi-commercial scale unit closely in coming months. This JV interest is a hidden asset that could be worth a multiple of Black Range’s entire market cap.
TER: You follow some potash stocks as well. Do you consider potash a way to play emerging economies? What are you projecting for that commodity?
PE: Potash has solid long-term fundamentals. Like uranium, it’s an essential commodity with few if any substitutes. It is a play on an emerging middle class in developing economies. But there’s a domestic angle as well: The U.S. imports 90% of the potash that it consumes. Passport Potash Inc. (PPI:TSX.V; PPRTF:OTCQX), located in Arizona, will be producing 2 million tons of potash per year, which could greatly help the U.S. reduce its dependency on foreign-sourced potash. Passport’s delivered costs will be untouchable west of the Mississippi, and Passport has easy access to both West Coast and Gulf of Mexico ports for exports to Asia.
Passport has one of the best potash projects in the world, yet the company’s market cap is a fraction of global junior peers, despite the fact that Passport released a preliminary economic assessment with a very robust, 27% after-tax internal rate of return. As Passport continues to derisk its project, its valuation could double or triple and still be just half that of junior potash peers like Karnalyte Resources Inc. (KRN:TSX) or Elemental Minerals Ltd. (ELM:TSX; ELM:ASX; EMINF:OTCPK). Passport is in active discussions with multiple strategic investors and offtake partners. Within the next six months, there’s a good chance that Passport will execute a strategic investment and/or offtake agreement, which will further derisk the story.
TER: Let’s move on to oil. The oil price is holding above $100 per barrel. Do you think that’s a sustainable price? What oil price do you use to evaluate an oil company’s worth or upside potential or downside risk?
PE: I never try to predict commodity prices. I just try to pick the companies with the best fundamentals. Commodity prices can rise and fall well beyond what known fundamentals would suggest. Who would’ve thought that oil prices would be up 19% year-to-date while silver is down 35%, gold down 23% and copper down 15%?
As for oil companies, I’m very excited about Zodiac Exploration Inc. (ZEX:TSX.V), which is an example of an oversold, underfollowed, misunderstood Venture Exchange-traded stock. It’s trading at $0.06 per share but it’s probably worth at least $0.20 per share. Zodiac has zero debt and $18M in cash, equal to about $0.05/share. The company controls 78,000 net acres of highly prospective oil properties in California. In late 2012, a private company named Aera Energy LLC executed a JV with Zodiac on about 20,000 acres of Zodiac’s holdings. Aera has committed to pay 100% of the cost of two vertical and two horizontal wells, which in total are expected to cost $50–60M, in order to earn a 50% interest in the 20,000 acres. This is a huge vote of confidence in Zodiac’s assets and implies a valuation for Zodiac that is far greater than what the market’s ascribing to it right now.
The icing on the cake is that Zodiac has tax pools that are conservatively worth $0.04 per share. Therefore, the per-share value of the company’s cash and tax pools are worth considerably more than the current stock price, and investors still get 78,000 highly prospective acres for free. The recent announcement that Western Energy Production LLC is pooling 10,000 acres with Zodiac is further evidence of increased activity and interest in Zodiac’s holdings. Majors in the region, including Exxon Mobil Corp. (XOM:NYSE), Royal Dutch Shell Plc (RDS.A:NYSE; RDS.B:NYSE), Chevron Corp. (CVX:NYSE) and Occidental Petroleum Corp. (OXY:NYSE) are well aware of Zodiac. Zodiac is in discussions with multiple parties regarding further development activities.
Pyramid Oil Co. (PDO:NYSE.MKT) is also located in California, which many people may not realize is one of the larger oil-producing states. Pyramid has $6M dollars in cash and no debt. It has fewer than 5M shares outstanding. This company is cash-flow positive and has been in existence for over 100 years—since 1909. The company is exploring multiple corporate initiatives right now to enhance shareholder value. Pyramid Oil is a well run, successful exploration and production play.
TER: You also follow the natural gas space. What companies are you keeping tabs on?
PE: CBM Asia Development Corp. (TCF:TSX.V) is a junior coalbed methane play in Indonesia. Some shareholders are suffering from investor fatigue, but the underlying fundamentals remain fantastic. CBM Asia is one of a few juniors surrounded by Exxon, Total (TOT:NYSE), BP Plc (BP:NYSE; BP:LSE), Chevron and others. Indonesia is perhaps the single best market for coalbed methane in the world. CBM’s land grab over the past several years could pay off big as soon as this year.
There’s a common perception that natural gas prices are low because in the U.S. and Canada they’re low, but in most places around the world, especially Europe and Asia, they’re actually two to four times higher. That makes CBM Asia a much more compelling play than a lot of domestic natural gas stocks. In Asia, prices are anywhere from $6–12 per thousand cubic feet ($6–12/Mcf) compared to the U.S., where they’re currently about $3.5/Mcf.
TER: There’s a lot of discussion about coal becoming less competitive as a U.S. fuel source compared to cheap domestic natural gas. In an article you wrote last December, you argued that the coal industry would probably never return to its 2011 highs. Has your outlook changed?
PE: Like it or not, coal will be with us for the next several decades. Some places around the world are increasing coal-fired electricity generation faster than other sources of energy. In the U.S., coal-fired power generation has fallen from about 50% of the total mix to about 40% over the past five years, largely due to low natural gas prices. But while coal use is in decline in the U.S., in China and India it’s still increasing at a fairly good clip. Coking coal is a bit different. It’s somewhat viewed as a necessarily evil because it’s used for making steel and there are few substitutes for coking coal in blast furnaces. But the amount of coking coal used globally is a small fraction of that of thermal coal that’s used to generate electricity.
TER: You cover Celsius Coal Ltd. (CLA:ASX), which has a coking coal project. What’s the story there?
PE: Celsius Coal is a junior that is in the right place at the right time with its Uzgen Basin coking coal project in the Kyrgyz Republic. Historical drilling shows the deposit hosts very high-quality coking coal, which is important because most coking coal around the world is lower quality and subject to greater price volatility. Because the project is located only a few hundred kilometers from the Chinese border, it has a captive regional market in western China.
Celsius will not be impacted by highly volatile seaborne coking coal prices. Its customers will benefit from stability, security of supply and reduced delivery times by choosing Celsius Coal versus other coking coal producers thousands of kilometers away. Celsius is lucky to have strong financial backing and a loyal shareholder base.
TER: Let’s conclude with your take on graphite. Last year was a rollercoaster ride for investors. What do you see as the demand drivers?
PE: It’s true that graphite has been a wild ride. The key to the story is that graphite prices have settled in well above historical levels. Demand drivers include the proliferation of electric vehicles. Tesla Motors, for example, uses batteries that contain up to 100 kilograms of graphite. Lithium-ion batteries will continue to be the main driver for graphite demand. Of course, Tesla’s current run rate of automobiles is not significant in a global context, but Tesla and many other electric car manufacturers popping up around the world will certainly move the needle in coming years.
TER: What companies are you following in that space?
PE: The lesson we learned last year is that the first few companies to get to market will enjoy strong pricing and strong demand. Dozens of graphite juniors are in the race, but less than half will finish. Big North Graphite Corp. (NRT:TSX.V) is not as big as its name suggests, but it should be cash-flow positive and selling graphite within six months. The company is aggressively pursuing an existing amorphous graphite region in Mexico. While better known, flake graphite plays could reach production within five years, Big North could be selling thousands of tons in Mexico and the U.S. next year. In fact, on July 19, Big North announced that it has already mined and stockpiled 190 tons. This is a highly speculative small-cap company, but one that could really take off once meaningful production begins.
TER: Do you have any final advice for investors in the energy space?
PE: Patience will be rewarded. While many global stock markets are at near-term highs, true contrarians should be happy to walk away from those markets and hold a basket of juniors. If one picks a diversified basket of well-positioned juniors, returns should dramatically outperform indexes like the S&P 500. It’s just a matter of time.
TER: Thank you for taking the time to speak with us today.
PE: Of course, thank you for having me.
In 2011, Peter Epstein, CFA, left his senior analyst position at a $3B hedge fund and formed MockingJay Inc., a consultancy for companies in the natural resources space and an informal investment advisor to high net worth investors, family offices and funds. Epstein’s areas of expertise include uranium, coal, potash, gold and oil & gas. He has published hundreds of articles on investment sites such as Seeking Alpha, The Motley Fool and Au-Wire.com.
Want to read more Energy Report interviews like this? Sign up for our free e-newsletter, and you’ll learn when new articles have been published. To see a list of recent interviews with industry analysts and commentators, visit our Interviews page.
DISCLOSURE:
1) Rita Sapunor conducted this interview for The Energy Report and provides services to The Energy Report as an employee. She or her family own shares of the following companies mentioned in this interview: None.
2) The following companies mentioned in the interview are sponsors of The Energy Report: CBM Asia Development Corp., Passport Potash Inc., Strathmore Minerals Corp., Uranerz Energy Corp., Zodiac Exploration Inc. and Energy Fuels Inc. Streetwise Reports does not accept stock in exchange for its services or as sponsorship payment.
3) Peter Epstein: I or my family own shares of the following companies mentioned in this interview: Passport Potash Inc., Big North Graphite Corp., Energy Fuels Inc., CBM Asia Development Corp., Black Range Minerals Ltd., Zodiac Exploration Inc., Celsius Coal Ltd. and Pyramid Oil Co. I personally am or my family is paid by the following companies mentioned in this interview: None. My company has a financial relationship with the following companies mentioned in this interview: Black Range Minerals Ltd. I was not paid by Streetwise Reports for participating in this interview. Comments and opinions expressed are my own comments and opinions. I had the opportunity to review the interview for accuracy as of the date of the interview and am responsible for the content of the interview.
5) Interviews are edited for clarity. Streetwise Reports does not make editorial comments or change experts’ statements without their consent.
6) The interview does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports’ terms of use and full legal disclaimer.
7) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned and may make purchases and/or sales of those securities in the open market or otherwise.
Streetwise – The Energy Report is Copyright © 2013 by Streetwise Reports LLC. All rights are reserved. Streetwise Reports LLC hereby grants an unrestricted license to use or disseminate this copyrighted material (i) only in whole (and always including this disclaimer), but (ii) never in part.
Streetwise Reports LLC does not guarantee the accuracy or thoroughness of the information reported.
Streetwise Reports LLC receives a fee from companies that are listed on the home page in the In This Issue section. Their sponsor pages may be considered advertising for the purposes of 18 U.S.C. 1734.
Participating companies provide the logos used in The Energy Report. These logos are trademarks and are the property of the individual companies.
101 Second St., Suite 110
Petaluma, CA 94952
Tel.: (707) 981-8204
Fax: (707) 981-8998
Email: [email protected]